����⣺A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ����⻯��ΪH
2O��H
2S����DΪ��Ԫ�أ�EΪ��Ԫ�أ�ԭ������F������Ԫ�أ���FΪ��Ԫ�أ�A��D���γɵĻ���������¾�ΪҺ̬����AΪ��Ԫ�أ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ�BΪ̼Ԫ�أ�CΪ��Ԫ�أ����γ�10���ӷ��ӣ�����A��B������������֮����C��������������ȣ��������⣮
��AΪ��Ԫ�أ�BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ�FΪ��Ԫ�أ�
��1��������������֪��CΪN��FΪ��Ԫ�أ�ԭ�Ӻ�����17 �����ӣ���3�����Ӳ㣬�������7�����ӣ�λ�����ڱ���3���ڵڢ�A�壮
�ʴ�Ϊ��N����3���ڵڢ�A�壮
��2��BΪ̼Ԫ�أ�DΪ��Ԫ�أ�ֱ������̬������ B
2D
2����ΪC
2O
2���Ҹ�ԭ������㶼����8���ӽṹ��������̼ԭ����̼ԭ��֮���γ�2�Թ��õ��Ӷԣ�ÿ��̼ԭ����1����ԭ���γ�2�Թ��õ��Ӷԣ���C
2O
2����ʽΪ

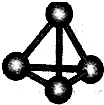
�������ʱ�ľ��������Ƿ��Ӿ��壮
�ʴ�Ϊ��

�����Ӿ��壮
��3����N
4ֻ��һ��Ԫ�أ����ڵ��ʣ��ʢٴ���
��N
4��P
4�����ף����γɷ��Ӿ��壬P
4��Է����������Ӽ�������ǿ��P
4�е�ߣ��ʢ���ȷ��
��lmol N
4����ת��ΪN
2���Ͽ���ѧ����������Ϊ167kJ��4=668kJ���γɻ�ѧ���ų�����Ϊ942kJ��2=1884kJ�����Է�Ӧ�ų�����Ϊ1884kJ-668kJ=1276kJ���ʢ۴���
��N
4��N
2���ǵ�Ԫ����ɵĽṹ��ͬ�ĵ��ʣ���Ϊͬ�������壬�ʢ���ȷ��
��P
4�����ף�����Ȼ���ȶ��Բ�ʢݴ���
��N
4���ڷ��Ӿ��壬�ʢ���
��N
4��P
4 �����ף��ľ��嶼���ڷ��Ӿ��壬�ʢ���ȷ��
��ͬ���칹���о�����Ϊ�����N
4��N
2��Ϊͬ�������壬�ʢ����
��ѡ���ڢܢߣ�
��4��CΪ��Ԫ�أ�FΪ��Ԫ������Ԫ���γ�һ�ֻ�������ӣ���ԭ��������8���ӽṹ�������д���3��N-Cl����
��÷��ӵĽṹʽΪ

���백���ṹ���ƣ���ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ��

�������ͣ�
��5��A
2ED
3ΪH
2SO
3��A
2D
2��H
2O
2��H
2O
2������������Ϊ���ᣬ��Ӧ���ӷ���ʽΪH
2O
2+H
2SO
3=2H
++SO
42-+H
2O��
�ʴ�Ϊ��H
2O
2+H
2SO
3=2H
++SO
42-+H
2O��
��6��E��F�γɵĻ�����E
2F
2ΪS
2Cl
2����ˮ��ˮ�⣬��ռ�ṹ��H
2O
2��Ϊ���ƣ�
a��S
2Cl
2�Ŀռ�ṹ��H
2O
2��Ϊ���ƣ����ԽṹʽΪ��Cl-S-S-Cl����a��ȷ��
b��H
2O
2Ϊ����ҳ�νṹ��-O-H��������ƽ�棬�Ǽ��Է��ӣ�S
2Cl
2�Ŀռ�ṹ��H
2O
2��Ϊ���ƣ�Ϊ���м��Լ��ͷǼ��Լ��ļ��Է��ӣ���b����
c��E
2Br
2��E
2F
2�ṹ���ƣ������γɷ��Ӿ��壬��Է�������Խ���۷е�Խ�ߣ������۷е㣺E
2Br
2��E
2F
2����c��ȷ��
d��S
2Cl
2��ˮ�⣬��Ӧ2S
2Cl
2+2H
2O=SO
2��+3S��+4HF������������ԭ��Ӧ���ҵ���ת����ȣ����ܷ����÷�Ӧ����d��ȷ��
��ѡ��acd��
��7��Ԫ�صķǽ�����Խǿ�����ʵ�������Խǿ��������������ǿ���������������ԣ����Է�ӦC1
2+H
2S=S��+2HCl������˵��Cl�ķǽ����Ա�Sǿ��
�ʴ�Ϊ��C1
2+H
2S=S��+2HCl��

 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��



 �������ʱ�ľ��������Ƿ��Ӿ��壮
�������ʱ�ľ��������Ƿ��Ӿ��壮 �����Ӿ��壮
�����Ӿ��壮 ���백���ṹ���ƣ���ռ乹��Ϊ�����ͣ�
���백���ṹ���ƣ���ռ乹��Ϊ�����ͣ� �������ͣ�
�������ͣ�

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�











