
ij�о���ѧϰС��������롰�о�����ˮ��Ӧ���ù������ʵijɷ֡����ʼ������á�ʵ��̽��������ͬ����������⣺
��̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�顣
��1��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ����____________________________________��
��3��װ��E�е�������________________________________________________��
��̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
��4����Ӳ�ʲ�����B��ȴ��ȡ�������еĹ�����������_______��������Һ�ֳ����ݡ�
��5��һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ֣�ѡ����ţ���ͬ��Ϊ_______������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ_______��
��һ����Fe3O4 ��һ����Fe
��ֻ��Fe3O4 ��ֻ��Fe
��6����һ����_______�����������ƣ�����_______������֤����Һ�д���Fe2+��
��̽����������������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������
��7���Լ�b�Ļ�ѧʽ��_______��
��8�����㷴Ӧ��Bװ������Ԫ�ص���������Ϊ_______��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ѧϰС���ijƷ��������Ħ�����ɷּ��京����������̽����
������ϣ� ������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
��Ħ���������������Ķ��Լ��飺 ȡ����������Ʒ����ˮ���衢���ˡ�
��1���������м������NaOH��Һ�����ˡ�����������NaOH��Һ��Ӧ�����ӷ���ʽ��______________��
��2������1��������Һ����ͨ�����������̼���ټ������ϡ���ᡣ�۲쵽��������________________��
��������Ʒ��̼��ƵĶ����ⶨ��������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������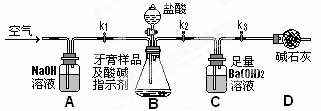
����ʵ����̻ش��������⣺
��3��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬����______
��4��C�з�Ӧ����BaCO3�Ļ�ѧ����ʽ��__________________________
��5�����и����ʩ�У�������߲ⶨȷ�ȵ���_________�����ţ���
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���壻
B���μ�����˹��죻
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��6��ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g������Ʒ��̼��Ƶ���������Ϊ_________��
��7��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С����ͨ������ʵ����̽��Na2CO3��NaHCO3�������ʵ����ʡ�
��1����ȡ���ֹ����2 g���ֱ��������С�ձ��У��ٸ��μ�10 mL ����ˮ���������¶ȱ仯�����������ܽ⣬���ָ������º���������Һ�и�����2�η�̪��Һ��
�� ����Na2CO3������ȫ�ܽ⣬��NaHCO3������ʣ�࣬�ɴ˵õ����� ��
�� ͬѧ�������ձ��л��۲쵽�������������У�ʢ��Na2CO3���ձ��г��ֵ������� ������ĸ��ţ���
A����Һ�¶��½� B����Һ�¶����� C�������̪���dz��ɫ D�������̪��ʺ�ɫ
��2����������ͼ��ʾ�ֱ����A��B���壬���ֹ���A���Ȳ�����������ʹ����ʯ��ˮ����ǣ���һ��ʱ�������ֱ���塣���û�ѧ����ʽ���ͳ���ʯ��ˮ�з��������� ��

��3������ͼ��ʾ�������������õ�װ��I��II�зֱ�����Լ����������ڵĹ���ͬʱ�����Թ��С�
���Թ��о��������壬 ���I����II�����ķ�Ӧ�̶ȸ�Ϊ���ҡ�
�� ��Ӧ����������������ͣ��ָ������¡�����˵����ȷ���� ��
A��װ��I����������ϴ� B��װ��II����������ϴ�
C������������������������� D�����������������ݹ������
��4�������ֹ���ֱ����Ƴ�0.5 mol��L-1����Һ��̽����0.5 mol��L-1CaCl2��Һ��Ӧ�����
| ʵ�鷽�� | Ԥ������ | Ԥ������ | ʵ�ʽ�� |
| ʵ��1����2 mL Na2CO3��Һ�еμ�1 mL 0.5 mol��L-1CaCl2��Һ | �а�ɫ ���� | Na2CO3��Һ�е�CO32-Ũ�Ƚϴ�����CaCl2������Ӧ�� | �а�ɫ���� |
| ʵ��2����2 mL NaHCO3��Һ�еμ�1 mL 0.5 mol��L-1CaCl2��Һ | �ް�ɫ ���� | NaHCO3��Һ�е�CO32-Ũ�Ⱥ�С��������CaCl2��Ӧ�� | �а�ɫ�������֣�ͬʱ����������ð���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Fe2����I�������ֳ����Ļ�ԭ�����ӡ�
��1����FeSO4��Һ�еμ���ˮ����Һ��dz��ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ ����KI��Һ�еμ���ˮ����Һ����ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽ�� ��
��2������FeSO4��Һ��KI��Һ����ˮΪ�Լ���֤I���Ļ�ԭ��ǿ��Fe2�������ʵ�鷽�����������ʵ�鲽�衢Ԥ������ͽ��ۡ�������ѡ�Լ���3 mol��L��1 H2SO4��0.01 mol��L��1 KMnO4��20% KSCN��3%H2O2��������Һ����ɫʯ����Һ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ2mLFeSO4��Һ��2mLKI��Һ������Թ��У��ٵμ�1��2����ˮ�� | �� |
| ����2��____________________________________ ____________________________________�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������Ũ�������ܷ����ۻ���ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣ʵ�������������Լ���0.01 mol/L ����KMnO4��Һ��0.1 mol/L KI��Һ��3��H2O2��Һ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��.������Һ�еĽ������ӿ��ܺ���Fe2+��Fe3+�е�һ�ֻ����֣�
��.���������п��ܺ���_________�е�һ�ֻ����֡�
| | ʵ����� | Ԥ������ | ���� |
| ��֤����� | ����٣�ȡ����0.01 mol/L����KMnO4��Һ������������Һ | | |
| ����ڣ�_________ | | ����Fe3�� | |
| ��֤����� | ����������ͨ������װ�� | | ������������ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧϰС����ͨ��ʵ��̽���̷���FeSO4��7H2O���ȷֽ�IJ��
��ʵ��ǰ����
��1�����۷��� С���Ա�������Ϸ�������Ϊ���зֽ���ﲻ���ܵ��� ��
a��Fe2O3��SO3��H2O b��Fe2O3��SO2��SO3��H2O
c��FeO��Fe2O3��SO2��SO3��H2O
��2���������� ��ѹ��SO3�۵�16��8�棬�е�44��6��
��ʵ��̽����
������Ͽ��ܵ���ϲ��룬��ѧϰС���������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
��3��ʵ�����
���������Ӻ��װ��A��B�����ԵIJ���Ϊ ��
��ȡһ�����̷���������A�У�ͨ��N2������װ���ڵĿ������ر�k���þƾ��Ƽ���
˫ͨ�ܡ�
�۹۲쵽A �й��������ɫ��B���Թ��ռ�����ɫҺ�壬C����Һ��ɫ��
�ܴ�A�з�Ӧ��ȫ����ȴ�����º�ȡ������Ӧ��������Թ��У����������ܽ⣬ȡ
�������뼸��KSCN��Һ����Һ���ɫ��
����Bװ�õ��Թ��е��뼸��BaCl2��Һ����Һ����ǡ�
( 4��ʵ��������
����1��B���ռ�����Һ���� ��
����2��C����Һ��ɫ������֪�������� ��
����3���ۺϷ�������ʵ��ۺܿ͢���֪�������һ����Fe2O3��
��ʵ�鷴˼��
��5����ָ����С����Ƶ�ʵ��װ�õ����Բ��㣺 ��
��6���ֽ��Ĺ����п��ܺ�������FeO��ȡ����ʵ����������ܽ�����Һ�������Թ��У�
ѡ��һ���Լ����𣬸��Լ�����ʵ��� ��
a����ˮ��KSCN��Һ b������KMnO4��Һ c�� H2O2 d�� NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ��Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����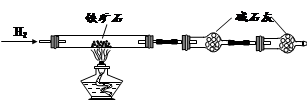
A B C
������ʯ�к������IJⶨ
�� ����ͼ��װ���������װ�õ������ԣ�
�� ��5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�� ����˵����ܿڴ�����ͨ��H2��____________����ȼA���ƾ���
�� ��ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ________________________________________________��
��2�����е�ȼA���ƾ���ǰ�������Ϊ��______________________________ ��
��3����÷�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ____________��
������ʯ�к������IJⶨ
��1�����������������__________________________________________��
��2����������õ��IJ����������ձ�������������ͷ�ιܡ�____________��
��3�������йز���IJ�����˵����ȷ����__________________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ���ɫ�仯��30s����Һ���ָ�ԭ������ɫ�ٶ���
��4�����ζ�����������0.5000mol��L?1KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ____________��
���ɢ���������������ʯ������������Ļ�ѧʽΪ ��
��μ�����ҺA���Ƿ���Fe2+__________����ѡ����ĸ����
A.�ȼ�KSCN��Һ���ټ���ˮ B.��NaOH��Һ C.��K3[Fe(CN)6]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ�о���ѧϰС���ͬѧ���������»�ѧʵ�飺�����������ڿ�����ȼ�գ�Ȼ�������ù�������м���һ��������ˮ���˹����з�Ӧ�ų��������ȣ����ҷų��г�ζ�����塣
��1����ͬѧ������������ѧϰ��˼�룬Ca��Mg��ͬһ����Ԫ�أ���ѧ���ʾ���һ���������ԡ�
��д��Ca�ڿ�����ȼ�շ�����Ӧ�Ļ�ѧ����ʽ�� ��
��2����ͬѧ�����Ca�����ʱ�Na���ã��ڿ�����ȼ�ջ�Ӧ��CaO2���ɣ���д��ȼ�պ���������ˮ��Ӧ�ų�����Ļ�ѧ����ʽ�� ��
��ͬѧ�����ʵ��ķ���̽���ų���ζ����ijɷݣ�
���������ϡ�1��CaO2��ˮ��Ӧ����H2O2��H2O2���ܻ�ֽ����һ������O3
2������������õij����ⶨ��������ԭ��Ϊǿ������������O3����⻯�أ�KI��ˮ��Һ��Ӧ��������⣨I2����������ԭΪ��������ӦʽΪ��O3+2KI+H2O��O2+I2+2KOH ��������衿����1���ó�ζ����ֻ��NH3��
����2���ó�ζ����ֻ�� ��
����3���ó�ζ���庬�� ��
����Ʒ�������ʵ��̽����
��3�����ڼ���l����Сͬѧ���������ʵ�鷽����������ʵ�顣���ڴ���ϰ��±���ʽ�����ص�ʵ��������衢Ԥ�������ۣ�������ѡ����
����ѡʵ���Լ�������ɫʯ����ֽ����ɫʯ����ֽ��pH��ֽ������һKI��Һ������ˮ�����ʵ�鷽��֤����ļ��裬�����±���ʽд��ʵ��������衢Ԥ������ͽ��ۡ�
| ʵ����� | Ԥ������ͽ��� |
| ȡ������Ӧ��������Թ��У� �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣����ʾ����ʯ������ʯ�����������ƵĻ�����������ˮ�Ͷ�����̼��
��Ҫʵ�鲽�����£��� ��ͼ��װ������������װ�õ�������
�� ��10.0 g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�� ����ʢ�м�ʯ�ҵ�U�ܵ��������õ�20.0g
�� �ӷ�Һ©������6mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ
�� �ӵ���A����������һ�����Ŀ���
�� �ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�22.0g
�� �ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊ22.2g
����պͻش����⣺
��1��װ���и����B������ _________________________�����û�����Ӹø���ܣ����ԵĽ�� (��ƫ�ߡ�ƫ�ͻ䣩��
��2���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ�� (��ƫ�ߡ�
ƫ�ͻ䣩��
��3������ݵ�Ŀ���� ___________________�����û�н��в���ݵIJ��������ԵĽ��________________����ƫ�ߡ�ƫ�ͻ䣩��
��4�������д������������Ϊ___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com