
 ͭ��һ����Ҫ����ɫ��������������;Խ��Խ�㷺����ش��������⣺
ͭ��һ����Ҫ����ɫ��������������;Խ��Խ�㷺����ش��������⣺���� ��1����ͭ��=$\frac{����ͭ�����ԭ������}{���ʵĺ�ͭ��}$��
��2�����ݻ�ѧʽCu2S����ͭԪ�ص�����������������һ��������Cu2S������ͭԪ�ص�������������ijԪ�ص��������������Ǹ�Ԫ�ص�������������ʵ�Ԫ��������֮�ȣ�
��3�����������֪��������������ͭ��N2H4����������ԭ��Ӧ����Cu2O��N2��
�ڹ�ҵ�ϳ��õĹ�Һ�����豸�����Ļ��Ϳ�ʽѹ�˻���
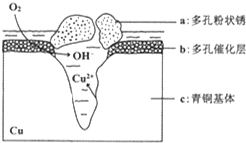
��4���ٸ���ͼ֪�������õ����������������ӡ�Cuʧ��������ͭ���ӣ�����������ʴ����Cu��������
��Cl-��ɢ���ڣ�����������Ӧ���������Ӧ�����������ɶ��״��Cu2��OH��3Cl������������ͭ���ӡ��������������������ӣ����Ը����ӷ�ӦΪ�����ӡ�ͭ���Ӻ����������ӷ�Ӧ����Cu2��OH��3Cl������
��� �⣺��1��a��Cu2S�к�ͭ��Ϊ$\frac{128}{160}$=0.8��b��Cu5FeS4�к�ͭ��Ϊ$\frac{320}{504}$=0.63��c��Cu2��OH��2CO3�к�ͭ��Ϊ$\frac{128}{221}$=0.58��d��CuFeS2�к�ͭ��Ϊ$\frac{64}{184}$=0.35��
�ʴ�Ϊ��a��
��2��Cu2S��ͭԪ�ص���������=$\frac{64��2}{160}$��100%=80%����X�ֺ�Cu2S 32%��ͭ��ʯ�к�ͭԪ������=Xt��80%��32%=796��t���X=3109.4��֣�
�ʴ�Ϊ��3109.4��
��3�����������֪��������������ͭ��N2H4����������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ4CuSO4+N2H4+8KOH$\frac{\underline{\;90��\;}}{\;}$2Cu2O+N2��+4K2SO4+6H2O��
�ʴ�Ϊ��4CuSO4+N2H4+8KOH$\frac{\underline{\;90��\;}}{\;}$2Cu2O+N2��+4K2SO4+6H2O��
�ڹ�ҵ�ϳ��õĹ�Һ�����豸�����Ļ��Ϳ�ʽѹ�˻���
�ʴ�Ϊ��AC��
��4���ٸ���ͼ֪�������õ����������������ӡ�Cuʧ��������ͭ���ӣ�����������ʴ����Cu����������c�Ǹ���������������ԭ��Ӧ���缫����ʽΪO2+2H2O+4e-�T4OH-��
�ʴ�Ϊ��c��O2+2H2O+4e-�T4OH-��
��Cl-��ɢ���ڣ�����������Ӧ���������Ӧ�����������ɶ��״��Cu2��OH��3Cl������������ͭ���ӡ��������������������ӣ����Ը����ӷ�ӦΪ�����ӡ�ͭ���Ӻ����������ӷ�Ӧ����Cu2��OH��3Cl���������ӷ���ʽΪCl-+2Cu2++3OH-�TCu2��OH��3Cl����
�ʴ�Ϊ��Cl-+2Cu2++3OH-�TCu2��OH��3Cl����
���� ���⿼��Cu���仯��������ʡ�ԭ���ԭ����Ӧ�ã��������ʵ������Լ��绯ѧ��Ӧԭ����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeS��Ksp��CuS��Ksp | |
| B�� | �ﵽƽ��ʱc��Fe2+��=c��Cu2+�� | |
| C�� | �÷�Ӧƽ�ⳣ��K=$\frac{{K}_{sp}��FeS��}{{K}_{sp}��CuS��}$ | |
| D�� | ��Һ�м�������Na2S�������Һ��c��Cu2+����c��Fe2+�����ֲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϵͳ��������������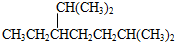 ��������2��3��5��5-�ļ�-4��4-���һ����� ��������2��3��5��5-�ļ�-4��4-���һ����� | |
| B�� | ���������ı��뱽������ȫȼ������������������� | |
| C�� | ����ױ���Ϊͬϵ�����ʹ���������Һ��ɫ | |
| D�� | �ṹƬ��Ϊ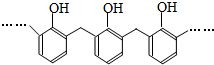 �ĸ߾���䵥���Ǽ�ȩ�ͱ��� �ĸ߾���䵥���Ǽ�ȩ�ͱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | I A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | F | H |
 ��
��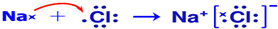
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ��� | / |
| ����2��ȡ��������1��Һ���Թ��У��μ�ϡ���� | ���ɰ�ɫ������˵�����Լ��к���Ba2+ |
| ����3��ȡ��������1�еij��������Թ��У��� | ����˵�����Լ��к���BaCO3 |
| ����4���� | �� |
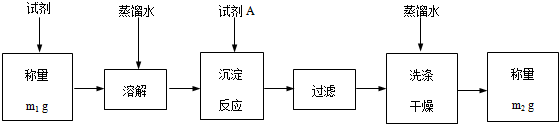
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/��L•min�� | B�� | 0.2mol/��L•min�� | C�� | 0.3mol/��L•min�� | D�� | 0.4mol/��L•min�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȶ��ԣ�HF��HCl��HBr��HI | B�� | ���ԣ�H4SiO4��H3PO4��H2SO4��HClO4 | ||
| C�� | ���Ӱ뾶��Al3+��Mg2+��Na+��F- | D�� | ���ԣ�Al��OH��3��Mg��OH��2��Ca��OH��2��KOH |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com