
(13��)��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�����
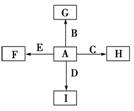
��֪��
�ٵ�����ֻ��AΪ�������ڴ�����߿��ͷŵ��������������ijȻ�ɫ��������ѧ���ɴ˿�ȷ������ڿ��е�λ�ã�B��C��DΪ���壬EΪ���壻C��EΪ��ɫ���ʡ�
��B��D�������ɻ�����J��A��J��Ӧ����D����һ�ֻ�����K��C��K��Ӧ�IJ��ﺬƯ��Һ����Ч�ɷ֣�F��G��I��ˮ��Һ�ʼ��ԡ�
�ش����⣺
(1)������K�ĵ���ʽΪ________��
(2)F��ˮ��Һ�ʼ��Ե�ԭ��(�����ӷ���ʽ��ʾ)_____________________
______________________________________________________________��
д��I��J��Ӧ�Ļ�ѧ��Ӧ����ʽ_________________________________
______________________________________________________________��
(3)ʵ�����Ʊ�C�����ӷ�Ӧ����ʽΪ_____________________________��
(4)D��C��ȼ�չ۲쵽��������_________________________________��
(5)������B��D���ɻ�����J�ķ�Ӧ�Ƴ�ȼ�ϵ�أ���1 g D��B��ȼ��������̬Jʱ���ų�120.9 kJ����������֪1 mol J ������ʱ����44.0 kJ��д����ʾD��ȼ���ȵ��Ȼ�ѧ����ʽ_______________________________________
_______________________________________________________________��
����K���������Һ��д����ȼ�ϵ�صĸ����ĵ缫��Ӧ����ʽ_________________________________________________________________��
�����������ɽ���A�ڴ�����߿��ͷŵ��������������ijȻ�ɫ�������ж�A����Ϊ�����ƣ�EΪ��ɫ���壬��EΪ������FΪ���ƣ�CΪ��ɫ���嵥�ʣ���C��K��Ӧ�IJ��ﺬƯ��Һ����Ч�ɷ֣���CΪ������KΪ�������ƣ��ɴ˿���֪B��D��J�ֱ�Ϊ������������ˮ����IΪ�⻯�ơ�GΪ�����ƻ�������ƣ�HΪ�Ȼ��ơ�
(5)1 g H2��O2��ȼ��������̬ˮ�ų�120.9 kJ����������1 mol H2��O2��ȼ������1 mol��̬ˮ�ų�241.8 kJ��������1 mol��̬ˮҺ��ʱ�ַų�44.0 kJ��������1 mol H2��O2��ȼ������1 molҺ̬ˮ���ų�285.8 kJ�������������Ȼ�ѧ����ʽΪ��H2(g)��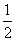 O2(g)===H2O(l)����H����285.8 kJ/mol��д�缫����ʽʱ��ע����������ҺΪ������Һ��
O2(g)===H2O(l)����H����285.8 kJ/mol��д�缫����ʽʱ��ע����������ҺΪ������Һ��
���𰸡���(1) 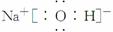
(2)S2����H2OHS����OH����NaH��H2O===H2����NaOH
(3)MnO2��4H����2Cl�� Mn2����Cl2����2H2O
Mn2����Cl2����2H2O
(4)��ɫ����
(5)H2(g)��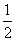 O2(g)===H2O(l)����H����285.8 kJ/mol
O2(g)===H2O(l)����H����285.8 kJ/mol
2H2��4e����4OH��===4H2O



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�ڻ�������������ʮ����Ҫ�����ã��绯ѧ���ⷨ����������ˮ�������ε���Ⱦ���绯ѧ����NO3����ԭ��ͼ��ʾ������˵������ȷ���ǣ�(����)

A��AΪ��Դ����
B������������
C������������ת����2mol���ӣ���Ĥ������Һ�������仯��
(��m��m��)Ϊ10.4��
D��������ӦʽΪ��2NO3����6H2O��10e����N2����12OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ܷ�����ȥ��Ӧ�������ܷ�����������Ӧ����(����)
A�� B��(CH3)2CHOH
B��(CH3)2CHOH
C��CH3CH2C(CH3)2CH2OH D��CH3CH2C(CH3)2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��������㴼��������ʳ���㾫����ṹ������ʾ��

(1)�㴼�ķ���ʽΪ________�������ܷ������л���Ӧ������________��(�����)
��ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ���ܾۺϷ�Ӧ
��������Ӧ����ˮ�ⷴӦ
(2)�л����(C13H18O2)��һ�����ϣ���ϳ�·����ͼ��ʾ�����м���Է�������Ϊ88�����ĺ˴Ź���������ʾ��3��壬��Ϊ�㴼��ͬϵ�

��֪��R��CH��CH2 R��CH2CH2OH��
R��CH2CH2OH��
��A������________��
��C�����Ƽ���Cu(OH)2��Ӧ�Ļ�ѧ����ʽ________________________
______________________________________________________________��
�۱���������������һ�������£�1 mol D���Ժ�2 mol H2��Ӧ�����ң�D���Է���������Ӧ����D�Ľṹ��ʽΪ______________________________��
�ܼ����ҷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________
_____________________________________________________________��
�ݼ�ͬ���칹���к��С��������ṹ�Ĺ���___________________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A��Na��Al��Fe����������һ����������ˮ��Ӧ������H2�Ͷ�Ӧ�ļ�
B��Ư�ۺ�����������������ˮ�Ĵ��������ߵ�����ԭ������ͬ
C����SO2ͨ��Ca(ClO)2��Һ������CaSO3����
D��������ͭ��Ũ���ᷴӦ�����ɵ�����ֻ��NO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ����ѡ��5���л���ѧ����](15��)
ij����W�Ľṹ��ʽ��ͼ��ʾ��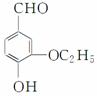
�밴Ҫ��ش��������⣺
(1)�л���W�к������������ŵ�����Ϊ________��
(2)���л���W��Ϊͬ���칹�壬����NaHCO3��Һ��Ӧ���ұ�����ֻ��һ��ȡ��������_________________________________________________��(����R��O��R�估R��O��COOH�ṹ��R��R���������)��
(3)�������������(2)ͬ���칹���е�һ�֣��ɷ�������ת����
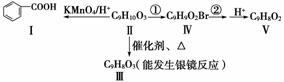
��֪���뱽��ֱ��������̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ���
��д����������к��������ŵĽṹ��ʽ��________��
���ɻ������Ҳ��ֱ�����ɻ����������Ʒ�Ӧ�ٺ͢ڵ�Ŀ����__________________________________________________________________��
��д�����з�Ӧ�Ļ�ѧ����ʽ��
��.�����___________________________________________________��
��.���������NaOHˮ��Һ���ȣ�_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з���ʽ��ʾ������һ���Ǵ�������� ( )
A . C5H10 B . C7H8O C . CH4O D . C3H8Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
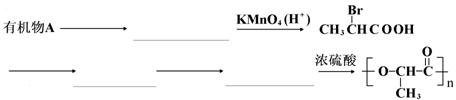
ij�л���A����C��H��O����Ԫ�أ��������ܶ�����ͬ������H2�ܶȵ�29������1.16g���л�����O2�г��ȼ�գ���������ͨ��������ʯ�ң���ʯ������3.72g����֪���ɵ�CO2��H2O�����ʵ���֮��Ϊ1:1 ��
��1���л���A�ķ���ʽ
��2�����л���ĺ��������ʾ�÷�������һ���ǻ���������״�ṹ����д��A�Ľṹ��ʽ ��
 |
��3����֪������ ��һ�ֺ���ǰ;�Ŀɽ���߷��Ӳ��ϣ����Ƴɴ��������
�������еĴ����ϴ�����������л���AΪԭ�Ϻϳɾ������·�ߣ����Լ���ѡ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ���Ȼ�菉���ľ�������֪������2�������Cs+���Ӻ˼��Ϊa cm���Ȼ�蘆�Ħ������ΪM��NAΪ�����ӵ����������Ȼ�菉�����ܶ�Ϊ
A��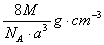 B��
B��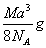
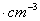
C��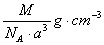 D��
D��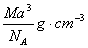
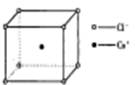
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com