
�л��������㴼��������ʳ���㾫����ṹ������ʾ��

(1)�㴼�ķ���ʽΪ________�������ܷ������л���Ӧ������________��(�����)
��ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ���ܾۺϷ�Ӧ
��������Ӧ����ˮ�ⷴӦ
(2)�л����(C13H18O2)��һ�����ϣ���ϳ�·����ͼ��ʾ�����м���Է�������Ϊ88�����ĺ˴Ź���������ʾ��3��壬��Ϊ�㴼��ͬϵ�

��֪��R��CH��CH2 R��CH2CH2OH��
R��CH2CH2OH��
��A������________��
��C�����Ƽ���Cu(OH)2��Ӧ�Ļ�ѧ����ʽ________________________
______________________________________________________________��
�۱���������������һ�������£�1 mol D���Ժ�2 mol H2��Ӧ�����ң�D���Է���������Ӧ����D�Ľṹ��ʽΪ______________________________��
�ܼ����ҷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________
_____________________________________________________________��
�ݼ�ͬ���칹���к��С��������ṹ�Ĺ���___________________�֡�
����������(1)�÷����к��б����ʹ��ǻ�����Ϸ��ӽṹ����֪�����л����ܹ�����ȡ����Ӧ���ӳɷ�Ӧ��������Ӧ����ȥ��Ӧ��
(2)�ӷ�Ӧ�п��Կ�����AΪϩ������˫��ˮ���������з�����Ӧ���ɴ�(B)����һ������Ϊȩ(C)��������Ϊ����ף�������Է�������Ϊ88��������֪��ΪC3H7COOH����˴Ź���������������壬���ṹ��ʽΪ(CH3)2CHCOOH�����ҷ���������Ӧ���ɱ������ݷ���ʽ���㣺C13H18O2��H2O��C4H8O2��C9H12O�����ҵķ���ʽ��C9H12O���䲻���Ͷ�Ϊ4����һ�����������ڱ���ֻ��������������֪�����������Ӷ�ȷ���ҵĽṹ��ʽΪ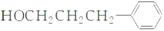 �����ƿ�֪D�Ľṹ��ʽ
�����ƿ�֪D�Ľṹ��ʽ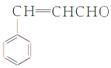
���𰸡���(1)C10H14O���٢ڢۢݡ�(2)��2����ϩ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ҵ����ӵ�˵����ȷ���ǣ�(����)
A�������й�����8�����Լ��� B�������в����Ǽ��Լ�
C��������ֻ���Ҽ�������������D�������к���1���м�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС���һ�������ǡ�NO2�ܷ�֧��ľ����ȼ�գ�������ʵ������û���ֳɵ�NO2���壬��С���ͬѧ���������������
����һ��ͼ����ʾ��4HNO3(Ũ) 4NO2����O2����2H2O
4NO2����O2����2H2O
��������ͼ����ʾ��2Cu(NO3)2 2CuO��4NO2����O2��
2CuO��4NO2����O2��

��˼�����ش���������
����һ��ʵ�鿪ʼ����ľ���ϵĻ�����Ϩ���ˡ�
��1����ͬѧ�ó���NO2����֧��ľ����ȼ�ա��Ľ��ۡ�����Ϊ��һ�����Ƿ���ȷ�� �����ȷ������ȷ����
��2����ͬѧ��Ϊʹľ���ϵĻ���Ϩ������DZ��������������ã�����Ϊ����ʹľ���ϵĻ���Ϩ������ʿ����ǣ� ���ѧʽ��
��������ʵ�鿪ʼ������ƿ�г�������ɫ����ʱ������ľ����ȼ�ˡ�
��3����ͬѧ�ó���NO2��֧��ľ����ȼ�ա��Ľ��ۣ���ͬѧ��Ϊ����������O2��������˲���֤��NO2�Ƿ�֧��ľ��ȼ�ա�����ͬѧ˵������Ϊ�����ǵ�ľ���ڿ����в��ܸ�ȼ������ ͬѧ��������������۵��ǶԵġ���������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1 mol 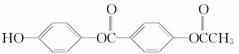 ��������NaOH��Һ��ַ�Ӧ�����ĵ�NaOH�����ʵ���Ϊ(����)
��������NaOH��Һ��ַ�Ӧ�����ĵ�NaOH�����ʵ���Ϊ(����)
A��5 mol B��4 mol C��3 mol D��2 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
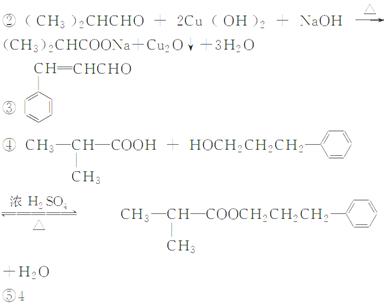
���������ijɷ��ж��֣����ᱽ���������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�

��1��д����Ӧ�ٵĻ�ѧ����ʽ�� ��
��2����Ӧ�۵ķ�Ӧ����Ϊ��
��3��д��C�еĹ����ŵ����ƣ�
��4�������٢ڢ�������Ӧ�У�ԭ�ӵ�����������Ϊ100%��������ɫ��ѧҪ��ķ�Ӧ�� ������ţ���
��5�����ᱽ�����кܶ�ͬ���칹�壬д����������Ҫ���ͬ���칹��ṹ��ʽ���ٺ��������ڱ�����ֻ��һ��ȡ����
��д����������ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ���������ֵ�������ж���ȷ����(����)
A�����³�ѹ�£�22.4 L CH4�к��е�C��H����Ϊ4NA
B��4.6 g��NO2��N2O4��ɵĻ�������к��е���ԭ����Ϊ0.3NA
C����0.2 mol H2SO4��Ũ����������Cu��Ӧ������SO2�ķ�����Ϊ0.1NA
D��1 mol Na������O2��ȼ������Na2O2����ʧȥ�ĵ�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��)��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�����
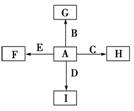
��֪��
�ٵ�����ֻ��AΪ�������ڴ�����߿��ͷŵ��������������ijȻ�ɫ��������ѧ���ɴ˿�ȷ������ڿ��е�λ�ã�B��C��DΪ���壬EΪ���壻C��EΪ��ɫ���ʡ�
��B��D�������ɻ�����J��A��J��Ӧ����D����һ�ֻ�����K��C��K��Ӧ�IJ��ﺬƯ��Һ����Ч�ɷ֣�F��G��I��ˮ��Һ�ʼ��ԡ�
�ش����⣺
(1)������K�ĵ���ʽΪ________��
(2)F��ˮ��Һ�ʼ��Ե�ԭ��(�����ӷ���ʽ��ʾ)_____________________
______________________________________________________________��
д��I��J��Ӧ�Ļ�ѧ��Ӧ����ʽ_________________________________
______________________________________________________________��
(3)ʵ�����Ʊ�C�����ӷ�Ӧ����ʽΪ_____________________________��
(4)D��C��ȼ�չ۲쵽��������_________________________________��
(5)������B��D���ɻ�����J�ķ�Ӧ�Ƴ�ȼ�ϵ�أ���1 g D��B��ȼ��������̬Jʱ���ų�120.9 kJ����������֪1 mol J ������ʱ����44.0 kJ��д����ʾD��ȼ���ȵ��Ȼ�ѧ����ʽ_______________________________________
_______________________________________________________________��
����K���������Һ��д����ȼ�ϵ�صĸ����ĵ缫��Ӧ����ʽ_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2008�걱�����˻�Ĺ�����Ӿ����(ˮ����)�Ľ���������Ĥ����ETFE���ò���Ϊ�ķ���ϩ����ϩ�Ĺ�����ķ���ϩҲ����������ϩ���۳ɾ�ȫ���ұ�ϩ������˵���������� ( )
A. �ϳ�ETFE���ϳɾ�ȫ���ұ�ϩ�ķ�Ӧ��Ϊ�Ӿ۷�Ӧ
B. ETFE�����п��ܴ��ڡ���CH2��CH2��CF2��CF2���������ӷ�ʽ
C.��ȫ���ұ�ϩ���ӵĽṹ��ʽ����Ϊ
D.�ķ���ϩ�����к��й��ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y�Ƕ���������Ԫ�أ�IΪ�����ܣ���λ�ǿ�kJ/mol�������±����������ж�����ȷ����
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4600 | 6900 | 9500 |
| Y | 580 | 1800 | 2700 | 11600 |
A��Ԫ��X�ij������ϼ���+1�� B��Ԫ��X�����γɻ�����ʱ����ѧʽ������XCl
C��Ԫ��Y�Ǣ�A���Ԫ�� D����Ԫ��Y���ڵ�3���ڣ���������ˮ���ҷ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com