
(9��)Ԫ����������ָ������ѧϰԪ�ؼ��仯����֪ʶ����Ҫ���ߡ���֪����Ԫ�أ�����Po���IJ���֪ʶ���±���ʾ��
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | -218.4 | 113 | | 450 |
| ���ʷе㣨�棩 | -183 | 444.6 | 685 | 1390 |
| Ԫ����Ҫ���ϼ� | -2 | -2,+4,+6 | -2,+4,+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
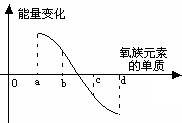
��1������113�棬С��450��(1��) ��2����2��+4��+6 (1��)
��3��H2Te��H2Se��H2S (2��) ��4����ԭ��(1��)��2H2Se+O2==2Se+2H2O(2��)
��5����(1��)����(1��)��
���������������1������������Ԫ���е����۵�ı仯��8O��52Te�����ߵĹ����еõ���
��2����ͬһ������Ԫ�ص���Ҫ���ϼ���ͬ���ڵ���Ҫ���ϼۿ����У�2��+4��+6��
��3����ͬһ�����У�Ԫ��ԭ������Խ��Ԫ�صķǽ�����Խ�����γɵ��⻯����ȶ���Խ��⻯��ˮ��Һ������Խǿ�����������ڵ��⻯��ˮ��Һ��������ǿ������˳����H2Te��H2Se��H2S��
��4����ͬһ�����У��γɵļ������ӵĻ�ԭ����ԭ�������������ǿ�����������н�ǿ�Ļ�ԭ�ԡ�
��5���ڴ˷�Ӧ�����зų�������Խ���⻯����ȶ���Խǿ���õ�a���⻯�����ȶ���d���⻯���ȶ������ɵá�
���㣺����������Ԫ��Ϊ���壬������Ԫ�������ɵ�Ӧ�á�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣���1�� �ѳ�ȥ����Ĥ����˿Ͷ�뵽ʢ��ϡ������Թ��У��������� �����ʱ仯�����ͼ��ʾͼ��t1~t2���ʱ仯����Ҫԭ���� ��t2~t3���ʱ仯����Ҫԭ���� ��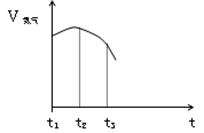
��2���ס��ҡ������������ֶ�����Ԫ�أ���ԭ����K����M���������ȣ���ԭ�ӵĺ� �����������ԭ�Ӻ����������1����ԭ�ӵ������������Ǵ�����������2������ԭ�Ӻ˵�����ȱ�ԭ�Ӻ˵������2����ش�
�ټĵ�����ˮ��Ӧ�����ӷ���ʽΪ ������Ԫ�������ڱ��е�λ��Ϊ ���۱�Ԫ�ص����������ĵ���ʽΪ ���ܼ��붡����Ԫ�ؿ���ɵľ���ǿ�����Ե������� �����ѧʽ��
��3��ijԪ��R��������ۺ��������������RO4����������̬�⻯��Ļ�ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�Ԫ�����ڱ���Ԫ����������ѧϰ���о�������ʵ�����к���Ҫ�����á��±��г��ˢ١������Ԫ�������ڱ��е�λ�á�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | | �� | | |
| 3 | �� | �� | �� | | | | �� | �� |
| 4 | �� | | | | | | �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ����ѡ��3�����ʽṹ�����ʡ�(15��)
±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǡ�
��1��±��Ԫ��λ��Ԫ�����ڱ���_________������ļ۵����Ų�ʽΪ____________________��
��2����һ��Ũ�ȵ���Һ�У���������Զ����ӵ�(HF)2��ʽ���ڵġ�ʹ�������ӵϵ���������________��
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_________��
| | �� | �� | �� | �� |
| ��һ������ (kJ/mol) | 1681 | 1251 | 1140 | 1008 |
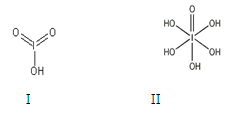
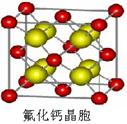
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����12�֣�
�ϳɰ����յ�һ����Ҫ������ͭϴ����Ŀ������ͭҺ[���������ͭ��I������ˮ]���������������в�����CO��CO2�����塣ͭҺ����CO�ķ�Ӧ�Ƿ��ȷ�Ӧ���䷴Ӧ����ʽΪ��
Cu(NH3)2Ac��CO��NH3 [Cu(NH3)3CO]Ac
[Cu(NH3)3CO]Ac
���������գ�
��1�����Ҫ���������Ӧ�ķ�Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ��_________����ѡ���ţ�
a.��ѹ b.����NH3��Ũ�� c.���� d.��ʱ���߲���
��2��ͭҺ�еİ������ն�����̼��д���÷�Ӧ�Ļ�ѧ����ʽ��
_________________________________________
��3������ͭҺ����CO��ͭҺ�����IJ������裨ע�����պ���������������
__________________________________________
��4��ͭҺ�����Ԫ���У�������Ԫ��ԭ�Ӱ뾶�Ӵ�С������˳��Ϊ____________________�����е�Ԫ��ԭ�����������Ų��Ĺ������ʽ��_________________________��ͨ���Ƚ�_____________���жϵ��������ַǽ���Ԫ�صķǽ�����ǿ����
��5����֪CS2��CO2���ӽṹ���ƣ�CS2�ĵ���ʽ��____________��CS2�۵����CO2����ԭ����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C����Ԫ�����ڱ��еĶ����ڷǽ���Ԫ�أ����ǵĺ˵������������Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2����Bԭ�ӵ������p����ĵ���Ϊ�����ṹ��C�ǵؿ��к�������Ԫ�ء�D��E�ǵ�������Ԫ�أ�Dԭ�Ӻ���������������1�����ӣ����������Ӿ�������Eԭ�Ӻ���δ�ɶԵ�������ͬ��������ࡣ���ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ�
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ �� A��C���⻯��е��С��ϵΪ������������������ԭ��Ϊ������������������������������������������������
����������������������������������������������������������������������������
��2��D��E��ԭ�ӻ��ȷֱ�Ϊ340 kJ��mol-1��400kJ��mol-1�������ǵ��۵㣺D E(�����������������="��" )��
��3������A2B2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�Ϊ�Ǽ��Է��ӣ�ÿ��ԭ������������������˵��ӣ���ṹʽΪ_____________��1mol�÷����к��� ������ĿΪ ��
������ĿΪ ��
��4����̬��ԭ�ӵ���Χ�����Ų�ʽΪ ��EO2Cl2�۵㣺��96 .5�棬�е㣺117�棬���̬EO2Cl2���� ���塣
��5��D���⻯��ľ���ṹ��ͼ��ʾ���仯ѧʽ�� ��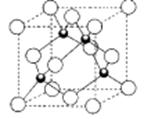
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[��ѧ��ѡ��3���ʽṹ������](15��)
±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǡ�
��1��±��Ԫ��λ�����ڱ���_________������ļ۵����Ų�ʽΪ____________________��
��2���ڲ�̫ϡ����Һ�У���������Զ����ӵ�(HF)2��ʽ���ڵġ�ʹ�������ӵϵ���������________��
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_________��
| | �� | �� | �� | �� | �� |
| ��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 | 900 |
 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ��:H5IO6_____HIO4����������� ������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ��:H5IO6_____HIO4����������� ������������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣� X��Y��Z��W��M��QΪԭ������������������ֶ�����Ԫ�أ������£�����Ԫ�صij�������������Ϊ���壬����Ϊ���塣X��M��W��Q�ֱ�ͬ���壬 X��ԭ�Ӱ뾶��С��Ԫ�أ���X����Y��Z��W�ֱ��γɵ�������ȵ����ַ��ӣ�W�ǵؿ��к�������Ԫ�ء��Իش��������⣺
(1)W��M��Q����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С������˳���� �� ��������������(��Ԫ�ط��ű�ʾ)��
(2)Ԫ��M��Q�����γɻ�����M2Q��д��M2Q�ĵ���ʽ ��
(3) Z��W��Q����Ԫ�صļ���̬�⻯�����ȶ�����ǿ���� ���е���͵��� �����÷���ʽ��ʾ��
(4) W��һ���⻯�ﺬ18������, ���⻯����QW2����ʱ����һ��ǿ��,�仯ѧ����ʽΪ������
(5)��X��Z��W��Q����Ԫ���е�����Ԫ�ؿ����һ��ǿ�ᣬ��ǿ���ϡ��Һ����ͭ��Ӧ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
(6)��X��Z��W��Q����Ԫ����ɵ��������Ӹ�����Ϊ1:1�Ļ�����A����֪A���������ᷴӦ�������壬�������������Ƶ�Ũ��Һ��Ӧ�������壬����ʹ��ˮ��ɫ��д��A����������������Һ�ڼ��������·�Ӧ�����ӷ���ʽ ��
(7)����ʽΪX2Y2W4�Ļ������뺬�����ʵ�����KOH����Һ��Ӧ��������Һ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊc(K+)�� �� ����������������������c(OH��)����2mL 0.1mol/L X2Y2W4����Һ��4mL0.01mol/L����KMnO4��Һ��ϣ����ֿ�ʼ��Һ��ɫ�仯�����ԣ�����ҺѸ����ɫ������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͼ������ͼ���dz��õĿ�ѧ�о�������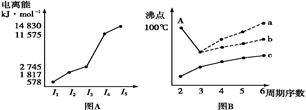
��.ͼ(A)�Ƕ�����ij����Ԫ��X�ĵ�������ʾ�������XԪ��λ�����ڱ��ĵ� �塣
ͼB���о�����Ԫ�ص��⻯��ķе�仯���ɵ�ͼ��,����c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ�
��.�±���Ԫ�����ڱ���һ����,�������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�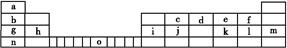
�Իش���������:
(1)��д��Ԫ��o����Χ�����Ų�ʽ: ��
(2)��jԭ�Ӹ�cԭ����1��1������϶��γɵľ���,���������뾧��j��ͬ����������۵���ߵ��� (�ѧʽ),�Դӽṹ�Ƕȼ��Խ���: ��
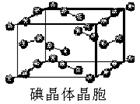
(3)i���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ,�侧����������ͼ����ʾ,ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��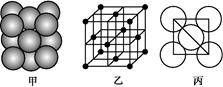
��ش�:������iԭ�ӵ���λ��Ϊ ,һ��������iԭ�ӵ���ĿΪ ���þ����Ŀռ�������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com