
 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��m![]() =m
=m![]() +m
+m![]() ?
?
n![]() (HCl)=n
(HCl)=n![]() (HCl)+n
(HCl)+n![]() (HCl)��?
(HCl)��?
����գ�?
��1��д�������ڢ�A���A��Ԫ���γɵ�����̼���ε����ƣ� ��?
��2������M![]() ��M
��M![]() ��M
��M![]() �ֱ��ʾA��B��C����Է�����������д��M
�ֱ��ʾA��B��C����Է�����������д��M![]() ��M
��M![]() ��M
��M![]() ���ߵ����ϵʽ ����������?
���ߵ����ϵʽ ����������?
��3��A����ȷѡ���� �֣��仯ѧʽΪ�� ��?
��4����A��BΪ��A��Ԫ�ص�̼���Σ�CΪ��A��Ԫ�ص�̼���Σ���A��B��C�Ļ�ѧʽ����������������������m![]() ��m=
��m=![]() 1�� ������2λС������?
1�� ������2λС������?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪�ֳ��������ѧУ��һ��ѧ��3���¿����ƻ�ѧ���⣨�������� ���ͣ������
A��B��C�Ƕ��������3��Ԫ�صĵ��ʣ��ס����dz����Ļ��������֮�������ͼ��ʾ��ת����ϵ����AΪ��ɫ���壬CΪ��ɫ���壬BΪ����ɫ���壬��ش�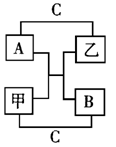
��1��д��ѧʽ��A B ��
��2��д����ʽ��C ��
��3��д��ѧ��Ӧ����ʽ��
A+�ס�B+��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com