
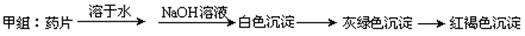 ����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������ 
 ����ʵ����֤��________________________________
����ʵ����֤��________________________________| ��� | V��KMnO4���� | V��KMnO4���� | V��KMnO4�� |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ���������mL�� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
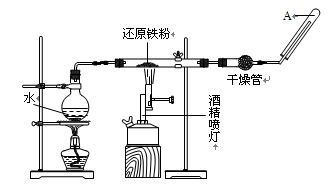
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
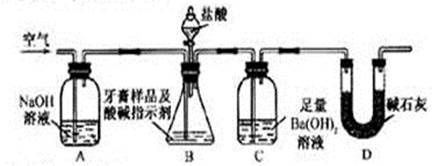
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƿʹ��ǰ����������ˮ |
| B�����ƹ����У�δ������ˮϴ���ձ��Ͳ����� |
| C����ת�ƹ���������Һ�������� |
| D������ȷ����Һ����̶������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ϩ��̼��������Ԫ�ص������ȡ�
ϩ��̼��������Ԫ�ص������ȡ�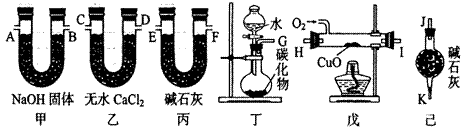
 ����ʽ�� ��
����ʽ�� �� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
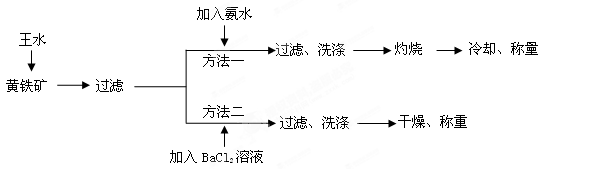
 S2+5HNO3+3HCl=FeCl3+2H2SO4+5NO��+2H2O
S2+5HNO3+3HCl=FeCl3+2H2SO4+5NO��+2H2O| A��NaOH | B��BaCl2 | C��HCl | D��Na2SO4 |
 ����ÿ�ʯ��FeS2������������________________��
����ÿ�ʯ��FeS2������������________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȡKMnO4��Ʒʱ�����ڱ���մ�˵������ˮ |
| B���ܽ����ʱ��Һ��ɽ� |
| C������ʱ��������ƿ�̶��� |
| D��ҡ�Ⱥ��Һ���½����ټ�ˮ���̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 Cl 2 + 5Cl 2�� + 8H2O
Cl 2 + 5Cl 2�� + 8H2O�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com