
��ҵ����CO2��H2��һ�������������·�Ӧ�ϳɼ״����ų��������ȣ�CO2(g)+3H2(g) CH3OH(g)+H2O(g)
��H1 �ش��������⡣
CH3OH(g)+H2O(g)
��H1 �ش��������⡣
��1����֪��2H2(g)+O2(g)=2H2O(g) ��H2
��Ӧ2CH3OH(g)+3O2(g)=2CO2(g)+4H2O(g) ��H= ���ú���H1����H2��ʾ��
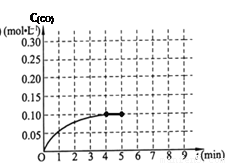
��2������Ӧ�¶����ߣ�CO2��ת���� (�������С�����䡱����
��3��д�������Ի����У��״�ȼ�ϵ���е�������Ӧ����ʽ
�������״���ԭ��H2�������·����Ƶã�CH4(g) + H2O(g)  CO(g)
+ 3H2(g)��һ���¶��£���2 mol CH4��4 mol
H2Oͨ���ݻ�Ϊ10L���ܱշ�Ӧ���У���Ӧ��CO�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺
CO(g)
+ 3H2(g)��һ���¶��£���2 mol CH4��4 mol
H2Oͨ���ݻ�Ϊ10L���ܱշ�Ӧ���У���Ӧ��CO�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺
��4����Ӧ���е�4���ӵ���ƽ�⡣�����ӷ�Ӧ��ʼ���ո�ƽ�⣬ƽ����Ӧ����v(H2)Ϊ ������˷�Ӧ�ڴ��¶��µ�ƽ�ⳣ�����ڴ����Ӧ�ķ�����д��������̣���
��5���ڵ�5����ʱ�����������˲����Сһ������ڵ�8����ʱ�ﵽ�µ�ƽ�⣨��ʱCO��Ũ��ԼΪ0.25 mol��L��1 ��������ͼ�л�����5���Ӻ�H2Ũ�ȵı仯���ߡ�
19����ָ���⣬����ÿ��2�֣���15�֣�
��1�� 3��H2-2��H1 ��2����С ��3��O2 + 4e- + 4H+ = 2H2O
��4��0.075 mol��L-1��min-1
���˿�4�֣�
CH4(g) + H2O(g)  CO(g) + 3H2(g)
CO(g) + 3H2(g)
��ʼ��mol��L-1�� 0.2 0.4
�仯��mol��L-1�� 0.1 0.1 0.1 0.3
ƽ�⣨mol��L-1�� 0.1 0.3 0.1 0.3
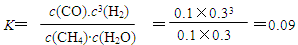
 ��5��
��5��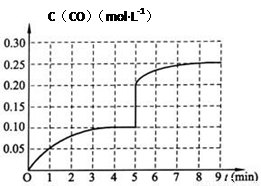
��3�֣�����Ե�1�֣��յ㡢ƽ̨��Ե�1�֡����ƶԵ�1�֡�5����ʱ������ֱ�����߲��۷֣�
��������
�����������1��������֪����������ʽ������ʽ��֪������ʽ����3����-2�������ã����Ԧ�H=3��H2-2��H1
��2����Ϊ��ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g)��һ�����ȷ�Ӧ�����Ը���ƽ���ƶ�ԭ�����¶����ߣ�ƽ�����ƣ�����CO2 ��ת���ʼ�С��
CH3OH(g)+H2O(g)��һ�����ȷ�Ӧ�����Ը���ƽ���ƶ�ԭ�����¶����ߣ�ƽ�����ƣ�����CO2 ��ת���ʼ�С��
��3����ص��ܷ�Ӧ����ʽ�Ѿ������������ܷ�Ӧʽ2CH3OH(g)+3O2(g)=2CO2(g)+4H2O(g)��O2 �����������ϼ۽��ͣ�����������������3��O2 ��12��e-�����������Խ��ʣ����Բ�����OH- ���뷴Ӧ�����Բ������H+ ��ͬʱ����ˮ�����Է�ӦʽΪ3O2 + 12e- + 12H+ = 6H2O�������ΪO2 + 4e- + 4H+ = 2H2O��
��4��ͼ���е���������CO��Ũ�ȣ�����Ҫ��������ƽ����Ӧ������Ҫ���ݷ���ʽ���б��Σ�����v(H2)= 3v(CO)=3��C/��t=0.3 mol��L-1/4 min =0.075 mol��L-1��min-1 ��
ƽ�ⳣ���ļ������Ϊ��
CH4(g) + H2O(g)  CO(g) + 3H2(g)
CO(g) + 3H2(g)
��ʼ��mol��L-1�� 0.2 0.4
�仯��mol��L-1�� 0.1 0.1 0.1 0.3
ƽ�⣨mol��L-1�� 0.1 0.3 0.1 0.3
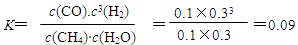
��5�������Сһ���ѹǿ�����Ҹ���ֵ�Ũ��˲������1��������ͼ��������0.1 mol��L��1˲������0.2 mol��L��1����ѹǿ����ƽ����ϵ����С��һ���ƶ�������CO��Ũ������ԼΪ0.25 mol��L��1 Ϊ�յ㡣
���㣺��Ҫ���鿼����ѧƽ���еļ��㡢ƽ���ƶ�ԭ������Ҫ��ѧ����ʽ�е��ʱ�ļ����Լ�ȼ�ϵ�ص缫��Ӧʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?̫ԭ��ģ������ȷ�ϣ�CO��SO2��NOx���ŷ�����ɴ�����Ⱦ����Ҫԭ��
��2011?̫ԭ��ģ������ȷ�ϣ�CO��SO2��NOx���ŷ�����ɴ�����Ⱦ����Ҫԭ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�̼�͵�����������ڹ�ũҵ����������������Ҫ�����á�
��1����CO2 ��H2 �ϳ�CH3OH������Ҫ���壬�ȿ��Խ���������⣬���ɽ����ԴΣ������֪�� +
=
+ 2
��H = ��725.5kJ��mol��1
2H2 (g)+O2(g) =2H2O(l) ��H = ��565.6kJ��mol��1��
��д����ҵ����CO2 ��H2 �ϳ�CH3OH(l)���Ȼ�ѧ����ʽ�� ��
��2��һ������ȼ�ϵ�أ�һ��ͨ�������һ��ͨ��CH3OH(g)��������Dz��������ƣ�Y2O3��������ﯣ�ZrO2�����壬������״̬���ܴ���O2�������ڸ����ڵ�����У�O2���� �� ���������)���ƶ�����ظ����缫��ӦΪ�� ������ ��
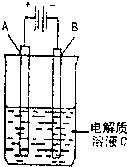
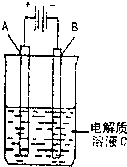
��3����ͼ��һ���绯ѧװ��ʾ��ͼ����CH3OH������ȼ�ϵ������װ�õĵ�Դ�����A�Dz��缫��B��ʯī�缫��C�� CuSO4 ��Һ��ͨ��һ��ʱ�����������Һ�м���8 g CuO�����ǡ�ÿ�ʹ��Һ�ָ������ǰ��Ũ�Ⱥ�pH������������ռ�����״���µ��������Ϊ ��
��4��������0.01mol��L��1�İ�ˮ�� = 1��10�� 6 �������Һ��pHΪ____����Һ�е����ʵ������������Ũ��ԼΪ ���� pH = 4��������ҺV1 L�� 0.01 mol��L��1��ˮV2 L��ϣ��������ҺpH = 7����V1 ��V2 �Ĺ�ϵΪ��V1 V2���>������<����=������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�ൺ�и���ͳһ������������ۺϣ���ѧ���֣� ���ͣ������
��14�֣�̼�͵�����������ڹ�ũҵ����������������Ҫ�����á�
��1����CO2 ��H2 �ϳ�CH3OH������Ҫ���壬�ȿ��Խ���������⣬���ɽ����ԴΣ������֪�� +
+
 =
=  + 2
+ 2 ��H = ��725.5
kJ��mol��1
��H = ��725.5
kJ��mol��1
2H2 (g)+O2(g) = 2H2O(l) ��H = ��565.6 kJ��mol��1 ��
��д����ҵ����CO2 ��H2 �ϳ�CH3OH(l)���Ȼ�ѧ����ʽ�� ��
��2��һ������ȼ�ϵ�أ�һ��ͨ�������һ��ͨ��CH3OH(g)��������Dz��������ƣ�Y2O3��������ﯣ�ZrO2�����壬������״̬���ܴ���O2�������ڸ����ڵ�����У�O2���� �� ���������)���ƶ�����ظ����缫��ӦΪ�� ������ ��
��3����ͼ��һ���绯ѧװ��ʾ��ͼ����CH3OH������ȼ�ϵ������װ�õĵ�Դ�����A�Dz��缫��B��ʯī�缫��C�� CuSO4 ��Һ��ͨ��һ��ʱ�����������Һ�м���8 g CuO�����ǡ�ÿ�ʹ��Һ�ָ������ǰ��Ũ�Ⱥ�pH������������ռ�����״���µ��������Ϊ ��

��4��������0.01
mol��L��1
�İ�ˮ�� = 1��10�� 6 �������Һ��pHΪ____����Һ�е����ʵ������������Ũ��ԼΪ
���� pH = 4��������ҺV1 L�� 0.01 mol��L��1
��ˮV2 L��ϣ��������ҺpH = 7����V1 ��V2 �Ĺ�ϵΪ��V1 V2
���>������<����=������
= 1��10�� 6 �������Һ��pHΪ____����Һ�е����ʵ������������Ũ��ԼΪ
���� pH = 4��������ҺV1 L�� 0.01 mol��L��1
��ˮV2 L��ϣ��������ҺpH = 7����V1 ��V2 �Ĺ�ϵΪ��V1 V2
���>������<����=������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��̫ԭ��ģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com