
���� ����һƿ��ɫ��Һ˵��һ������Cu2+��Fe3+��
�ٸ���Һ��ʹ��ɫʯ����ֽ���ֺ�ɫ��֤����Һ�����ԣ�˵����H+��һ������CO32-��
��ȡ��Һ������������ˮ��������Һ���ɼ���Һ������ɫ��֤��ԭ��Һ�к�I-����һ������Fe3+��
��ȡ��Һ�������Ȼ�����Һ���а�ɫ�������ɣ�����HNO3��������ܽ⣬֤��ԭ��Һ��һ����SO42-��һ������Ba2+��
��ȡԭ��Һ��������NaOH��Һ�ʼ��ԣ�������Һ���ȣ�����ɫ����ų�����������ʹʪ��ĺ�ɫʯ����ֽ������˵��һ����NH4+��
��� �⣺����һƿ��ɫ��Һ˵��һ������Cu2+��Fe3+��
�ٸ���Һ��ʹ��ɫʯ����ֽ���ֺ�ɫ��֤����Һ�����ԣ�˵����H+��һ������CO32-��
��ȡ��Һ������������ˮ��������Һ���ɼ���Һ������ɫ��֤��ԭ��Һ�к�I-����һ������Fe3+��
��ȡ��Һ�������Ȼ�����Һ���а�ɫ�������ɣ�����HNO3��������ܽ⣬֤��ԭ��Һ��һ����SO42-��һ������Ba2+��
��ȡԭ��Һ��������NaOH��Һ�ʼ��ԣ�������Һ���ȣ�����ɫ����ų�����������ʹʪ��ĺ�ɫʯ����ֽ������˵��һ����NH4+��
����������֪��
��1����Һ�п϶����ڵ�������NH4+��H+��I-��SO42-���ʴ�Ϊ��NH4+��H+��I-��SO42-��
��2����Һ�п϶������ڵ�������Ba2+��Cu2+��Fe3+��CO32-���ʴ�Ϊ��Ba2+��Cu2+��Fe3+��CO32-��
��3����Һ�в���ȷ���Ƿ���ڵ������У�K+��Cl-���ʴ�Ϊ��K+��Cl-��
���� ���⿼���˳������ӵļ��飬��Ŀ�Ѷ��еȣ�ע�����ճ������ӵļ��鷽������ȷ�������ӵ�������Ӧ���ܹ����ݷ�Ӧ�����ж���Һ�д��ڻ��߲����ڵ����ӣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3��17 | B�� | 6��8 | C�� | 12��9 | D�� | 8��16 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ������ | �����Լ� | �����Լ�������Ӧ�����ӷ���ʽ |
| A | K+��AlO2-��HCO3- | ����CO2 | 2AlO2-+CO2+3H2O�T2Al��OH�� 3��+CO32- |
| B | Ca2+��HCO3-��Cl- | ����NaOH��Һ | Ca2++2HCO3-+2OH-�T2H2O+CO32-+CaCO3�� |
| C | Fe2+��NO3-��NH4+ | NaHSO4��Һ | 3Fe2++NO3-+4H+�T3Fe3++NO��+2H2O |
| D | NH4+��Al3+��SO42- | ����Ba��OH��2��Һ | 2NH4++SO42-+Ba2++2OH-�TBaSO4��+2NH3•H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
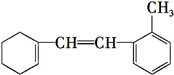
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AlCl3 | B�� | Al��OH��3 | C�� | Na2CO3 | D�� | Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
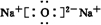 ��
��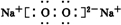 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����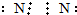 | B�� | ����H��N��H | ||
| C�� | ��������H+[��O��]2-H+ | D�� | �Ȼ���Na+[��Cl��]- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{1}{1}H$��$\frac{2}{1}H$��Ϊͬλ�� | B�� | ���ۺ���ά�ػ�Ϊͬ���칹�� | ||
| C�� | 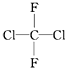 �� ��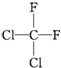 ����ͬһ������ ����ͬһ������ | D�� | C2H6��C4H10��Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ����� | ʵ��Ŀ�� | |
| A | ������Һ��ͨ�������̼ | ȷ���������Խ�̼���� |
| B | ��������۵ı��м�Ũ��ˮ | ��ȡ�屽 |
| C | �����Ƶ�������ͭ�м�����ȩ��Һ������ | ֤����ȩ�ɱ���ԭ |
| D | ��������������������Һ����һ��ʱ�䣬������ȴ��Ļ��Һ�еμ���������Һ | ����ˮ������е������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com