
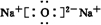 ��
��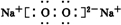 ��
������ ��A��Cͬ���壬ԭ�Ӱ뾶C��A����A��C���������8��Cԭ�Ӻ��ڵ�����������A��Bԭ�Ӻ��ڵ�������֮�ͣ��Ƴ�Bԭ�ӵ�������Ϊ8����BΪOԪ�أ�CΪ��������Ԫ�أ���Cԭ��������������Dԭ��������������3������D�����Ϊ1�����ӣ���C�����Ϊ3�����ӣ���D�������2�����ӣ���C�������6�����ӣ���C��B��ͬ����Ԫ���ˣ����������⣬��CΪAlԪ�أ�DΪNaԪ�أ�AΪBԪ�أ�����Ԫ���������ڱ��е�λ�ý��Ԫ�������ɵĵݱ���ɽ����⣮
��� �⣺��A��Cͬ���壬ԭ�Ӱ뾶C��A����A��C���������8��Cԭ�Ӻ��ڵ�����������A��Bԭ�Ӻ��ڵ�������֮�ͣ��Ƴ�Bԭ�ӵ�������Ϊ8����BΪOԪ�أ�CΪ��������Ԫ�أ���Cԭ��������������Dԭ��������������3������D�����Ϊ1�����ӣ���C�����Ϊ3�����ӣ���D�������2�����ӣ���C�������6�����ӣ���C��B��ͬ����Ԫ���ˣ����������⣬��CΪAlԪ�أ�DΪNaԪ�أ�AΪBԪ�أ�
��1�����ݷ�����֪��AΪ��Ԫ�أ�DΪ��Ԫ�أ�
�ʴ�Ϊ�����ƣ�
��2����B��D��ɵ����ֻ�����ֱ�ΪNa2O��Na2O2�����������ӻ��������ʽ�ֱ�Ϊ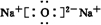 ��
��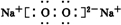 ��
��
�ʴ�Ϊ��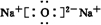 ��
��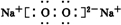 ��
��
��3��CΪAlԪ�أ���Ӧ��������ΪAl2O3��Ϊ���ӻ�����۵�ߡ�Ӳ�ȴ������ͻ���ϣ�Ҳ�����ڵ��ұ������ԭ�ϣ�
�ʴ�Ϊ�����ͻ���ϻ���ұ������
��4��A��C������������Ӧˮ����ֱ�ΪH3BO3��Al��OH��3������H3BO3ΪһԪ�ᣬAl��OH��3Ϊ���������������ǿ����H3BO3��
�ʴ�Ϊ��H3BO3��
��5��Al2O3Ϊ�������������NaOH��Һ��Ӧ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-�T2AlO+H2O��
�ʴ�Ϊ��Al2O3+2OH-�T2AlO+H2O��
���� ���⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�Ѷ��еȣ���ȷ�ƶ�Ԫ�ص�����Ϊ������Ĺؼ���ע����������Ԫ�����ڱ��ṹ��Ԫ�����������ݣ�


 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 200mL | B�� | 500 mL | C�� | 250 mL | D�� | 100 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ζ������ɫ����ʳƷ���Ӽ���������Խ��Խ�� | |
| B�� | �߲ˡ�ˮ��������ʳ��ά�����������������彡�� | |
| C�� | ά����c������������Ҫ���ܣ�ά����c������ˮ | |
| D�� | ��������������Ԫ�أ�ʳ�ε���Ҫ�ɷ���KCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ˮ����֭����ɰ�ǡ�ʳƷ���Ӽ� | |||||
| ��Ŀ | ���� | ά���� | ����ζ�� | �� | �� | �� |
| ÿ100g | 42.5g | 200mg | 100mg | 50mg | 90mg | 3.5mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O2�ĵ���ʽ�� | |
| B�� | �����ӵĽṹʾ��ͼ��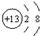 | |
| C�� | ������Ϊ35��������Ϊ45����ԭ�ӣ�${\;}_{35}^{45}$Br | |
| D�� | ${\;}_{6}^{14}$C��${\;}_{6}^{12}$C��Ϊͬ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X��YԪ�ص���������֮��Ϊ1 | |
| B�� | ��X+��Y2-����������Ӳ㣬��X2Y���������ӻ�����Ҳ�����ǹ��ۻ����� | |
| C�� | ��X+��Y2-������������8����ԭ�Ӱ뾶Y��X | |
| D�� | ��X+��Y2-������ͬ�ĵ��Ӳ�ṹ�������Ӱ뾶X+��Y2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���������ﶼ������������ | |
| B�� | �÷�Ӧ��NOx������������������ԭ��Ӧ | |
| C�� | ��x=2ʱ��ÿ����1molN2��ת�Ƶ�����Ϊ4mol | |
| D�� | �������뻹ԭ�������ʵ���֮��Ϊ1��1ʱ��NOx�е�Ԫ�صĻ��ϼ�Ϊ+2�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com