
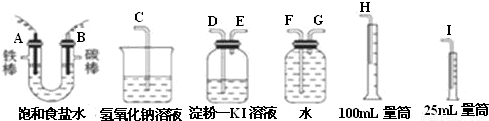
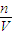 ������NaOH�����ʵ���Ũ�ȣ�
������NaOH�����ʵ���Ũ�ȣ� 2NaOH+H2��+Cl2������������H2�����Ϊ44.8mL0.002molʱ�������������Ƶ����ʵ���Ϊ0.004mol��������Һ��NaOH�����ʵ���Ũ��=
2NaOH+H2��+Cl2������������H2�����Ϊ44.8mL0.002molʱ�������������Ƶ����ʵ���Ϊ0.004mol��������Һ��NaOH�����ʵ���Ũ��=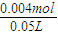 =0.08mol/L��
=0.08mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
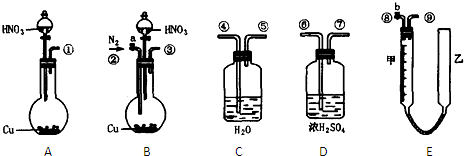
| V-11.2n |
| 33.6n |
| V-11.2n |
| 33.6n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
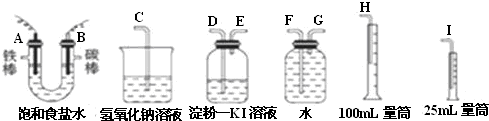
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
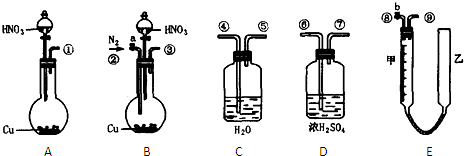
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
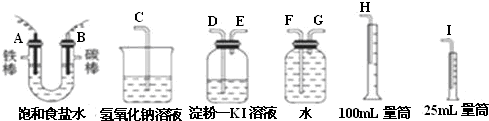
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com