
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
(1)��CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s)��3C(s)=2Fe(s)��3CO(g)����H1����489.0 kJ��mol��1
C(s)��CO2(g)=2CO(g)����H2����172.5 kJ��mol��1��
��CO��ԭFe2O3���Ȼ�ѧ����ʽΪ________________________________
(2)ijʵ�齫CO2��H2����һ��������ܱ������У������ֲ�ͬ�����·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ����ش��������⣺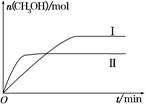
�ٸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK��________��
�����ߢ��Ӧ��ƽ�ⳣ����С��ϵΪK��________K��(����ڡ��������ڡ���С�ڡ�)��
������ͼa��b��c�����У�H2��ת�����ɸߵ��͵�˳����________(����ĸ)��
(3)�������������������£����������ѹ����ԭ����1/2����ԭƽ����ȣ������й�˵����ȷ����________(�����)��
a��������Ũ�ȼ�С
b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ�������
d������ƽ��ʱn(H2)/n(CH3OH)����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ϊ��������ʵʩ�ġ��������䡱�����յ�վ�����Ϻ����ı���ú��ʯ��Ϊ������Դ�ṹ����Խ�����л�����Ⱦ�����ش�
(1)Ŀǰ�Ϻ��ֳ��о�����ʹ�õ�ȼ����Ҫ�ǹܵ�ú�����ֶ���������
��ʼʹ�ö�����Ȼ����Ϊ����ȼ�ϣ��ܵ�ú������Ҫ�ɷ���CO��H2������
���࣬��Ȼ������Ҫ�ɷ���CH4������ȼ�յĻ�ѧ����ʽΪ��
2CO��O2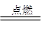 2CO2��2H2��O2
2CO2��2H2��O2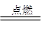 2H2O��CH4��2O2
2H2O��CH4��2O2 CO2��2H2O
CO2��2H2O
�������ϻ�ѧ����ʽ�жϣ�ȼ����ͬ����Ĺܵ�ú������Ȼ�������Ŀ�������ϴ�����������������ȼ�չܵ�ú����������������Ȼ������ߵĸĽ������������������(�����С��)���粻���Ľ����ܲ����IJ����������������������������������������
(2)�ܵ�ú���к��е����࣬�������⣬�����������顢���顢����ȣ����ǵ�ijЩ�������£�
| | ���� | ���� | ���� |
| �۵�/�� | ��183.3 | ��189.7 | ��138.4 |
| �е�/�� | ��88.6 | ��42.1 | ��0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��С�ձ������Լ20 g����ĥ�ɷ�ĩ��������������[Ba��OH��2��8H2O]����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����ձ��ڼ���Լ10 g�Ȼ�茶��壬�������ò�����Ѹ�ٽ��衣�Իش��������⣺
��1��д����Ӧ�Ļ�ѧ����ʽ��______________��
��2��ʵ��Ҫ�����ò�����Ѹ�ٽ����ԭ����_________________��
��3�����ʵ����û�п���������������ܵ�ԭ����__________________________�������������������ԭ��
��4�����û�п�����������������ǻ����Բ�ȡ��Щ��ʽ��˵���÷�Ӧ���ȣ�__________________________________________________________________________________________��������ַ�������
��5�������������˵����Ӧ��һ��________����ų��������ա��������ķ�Ӧ�����Ͽ��ɻ�ѧ��________����ų��������ա�������____________���>����<�����γ��»�ѧ��________������ա��ų�������������
��6���÷�Ӧ�ڳ����¾Ϳ��Խ��У�˵��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ�����ǰѲ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ��,Ҳ���Թ��㻯ѧ��Ӧ�ķ�Ӧ��(��H),��ѧ��Ӧ�Ħ�H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJ
| ��ѧ�� | Si��O | Si��Cl | H��H | H��Cl | Si��Si | Si��C |
| ����/kJ��mol-1 | 460 | 360 | 436 | 431 | 176 | 347 |
 ����ʾ�辧���е�һ��ԭ��,����������Ķ����á�
����ʾ�辧���е�һ��ԭ��,����������Ķ����á� ����ʾ����֮���ڵĹ�ԭ�ӡ�
����ʾ����֮���ڵĹ�ԭ�ӡ�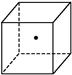
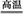 Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol��
Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų������ȡ���֪��0��4 molҺ̬����������˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256��652 kJ��������
��ش��������⣺
��1���÷�Ӧ���Ȼ�ѧ����ʽΪ_______________________________________________��
��2������֪H2O(l)=H2O(g)����H����44 kJ��mol��1����16 gҺ̬����˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������________ kJ��
��3���˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ��������������һ���ܴ���ŵ���________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ����ͼ��ʾ��
��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol���������)��
��1��д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ___________________________________��
��2��д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ_______________________________________________________________��
��3����ѧ�Ϲ涨�����γ�1 mol��ѧ�����ջ�ų���������Ϊ�û�ѧ���ļ��ܣ���λkJ��mol������֪�������ļ���Ϊd kJ��mol��1���������ļ���Ϊe kJ��mol��1����S8������������ļ���Ϊ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ϩ���ִ�ʯ�ͻ�����Ʒ������Ҫ�ĵ���֮һ���ڹ�ҵ�ϣ�����ϩ�����ұ���CO2
�������Ƶá��ܷ�Ӧԭ�����£�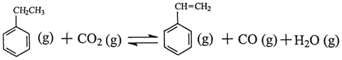 ��H
��H
�ش��������⣺
��1���ұ���CO2�����еķ�Ӧ�ɷ��������У�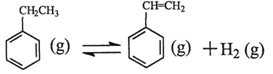 ��H1=��117.6kJ��mol��1
��H1=��117.6kJ��mol��1
H2 (g)��CO2 (g) CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
���ұ���ȡ����ϩ��Ӧ��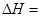 ��
��
��2�����¶�ΪT1ʱ���÷�Ӧ��ƽ�ⳣ��K=0.5mol/L����2L���ܱ������м����ұ���CO2����Ӧ��ijʱ�̲�û�����и���ֵ����ʵ�����Ϊ1.0mol��
�ٸ�ʱ�̻�ѧ��Ӧ ����ǡ����ǡ�������ƽ��״̬��
������������˵���ұ���CO2�ڸ������·�Ӧ�Ѵﵽƽ��״̬���� ������ȷ�𰸱�ţ���
a�������淴Ӧ���ʵı�ֵ�㶨 b��c(CO2)=c(CO)
c�����������ܶȲ��� d��CO2������������ֲ���
��������Ӧ��Ϊ��ѹ���������½��У��ﵽƽ��ʱ�����ұ������ʵ���Ũ�� ������ȷ�𰸱�ţ�
| A������0.5mol/L | B����0.5mol/L |
| C������0.5mol/L | D����ȷ�� |
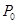 ����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�
����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�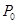 ��P�ı���ʽ��ʾ����
��P�ı���ʽ��ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ú������ҵ����һ����,��������ѧ֪ʶ,�����������: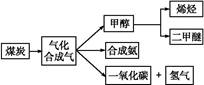
��.��֪�ò�ҵ����ij��Ӧ��ƽ�ⳣ������ʽΪ:K=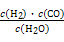 ,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:
,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:
����
��.������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�á���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ����(ѹ��2.0��10.0 MPa,�¶�230��280 ��)�������з�Ӧ:
��CO(g)+2H2(g) CH3OH(g)
CH3OH(g)
��H1="-90.7" kJ��mol-1
��2CH3OH(g) CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��H2="-23.5" kJ��mol-1
��CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)
��H3="-41.2" kJ��mol-1
(1)д������Ӧ����������Ӧ���ܷ�Ӧ���Ȼ�ѧ����ʽ: ��
(2)��ij�¶���,2 L�ܱ������з�����Ӧ��,��ʼʱCO��H2�����ʵ����ֱ�Ϊ2 mol��6 mol,3 min��ﵽƽ��,���CO��ת����Ϊ60%,��3 min��CO��ƽ����Ӧ����Ϊ��������������������ͬ����������ʼʱCO���ʵ���Ϊ4 mol,�ﵽƽ���CH3OHΪ2.4 mol,����ʼʱH2Ϊ��������mol��
(3)�����йط�Ӧ�۵�˵����ȷ��������������
A.������ɱ���ܱ�������,�ڷ�Ӧ�۴ﵽƽ���,����ѹ,��ƽ�ⲻ�ƶ����������ƽ����Է����������䡢��������ܶȲ���
B.��830 ��ʱ��Ӧ�۵�K=1.0,���ڴ���Ӧ���з�Ӧ�۵�K��1.0
C.ij�¶���,�����Ѵ�ƽ��ķ�Ӧ���м�������ʵ�����CO��H2O(g),��ƽ�����ơ�ƽ�ⳣ�����
(4)Ϊ��Ѱ�Һ��ʵķ�Ӧ�¶�,�о��߽�����һϵ��ʵ��,ÿ��ʵ�鱣��ԭ������ɡ�ѹǿ����Ӧʱ������ز���,ʵ������ͼ,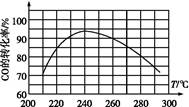
��COת�������¶ȱ仯�Ĺ����� ����
��ԭ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ҫ�Ļ�����Ʒ֮һ��
(1)�ϳɰ��õ��������Լ���Ϊԭ���Ƶá��йػ�ѧ��Ӧ�������仯����ͼ��ʾ��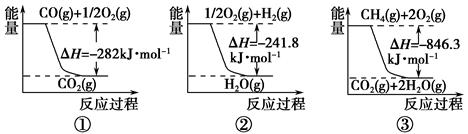
��Ӧ�٢ڢ�Ϊ________��Ӧ(����ȡ����ȡ�)��
CH4(g)��H2O(g)��Ӧ����CO(g)��H2(g)���Ȼ�ѧ����ʽΪ___________��
(2)�ð�����ȡ����[CO(NH2)2]�ķ�ӦΪ��2NH3(g)��CO2(g) CO(NH2)2(l)��H2O(g)��
CO(NH2)2(l)��H2O(g)��
��ij�¶��£����ݻ�Ϊ10 L���ܱ�������ͨ��2 mol NH3��1 mol CO2����Ӧ�ﵽƽ��ʱCO2��ת����Ϊ50%���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��______�����¶���ƽ�ⳣ��K�ļ�����Ϊ_____��
��Ϊ��һ�����CO2��ƽ��ת���ʣ����д�ʩ���ܴﵽĿ�ĵ���________��
| A�����NH3��Ũ�� | B������ѹǿ |
| C����ʱת�����ɵ����� | D��ʹ�ø���Ч�Ĵ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com