
������F��һ����Ҫ���л��ϳ��м��壬���ĺϳ�·�����£�
��1��������F�к��������ŵ������� �� ����B����C�Ļ�ѧ��Ӧ������ ��
��2��д��������C�����ᷴӦ�������Ļ�ѧ����ʽ��
��3��д��������B�Ľṹ��ʽ ��
��4��ij��������D��ͬ���칹�壬�ҷ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�д����
������Ľṹ��ʽ (��дһ��)��
��5�����������֪ʶ����������Ϣ��д���Ա���( )��CH2��CH2Ϊԭ���Ʊ�
)��CH2��CH2Ϊԭ���Ʊ�
�л��� 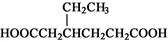 �ĺϳ�·������ͼ(���Լ�����)��
�ĺϳ�·������ͼ(���Լ�����)��
�ϳ�·������ͼʾ�����£�
H2C="=" CH2 CH3CH2Br
CH3CH2Br  CH3CH2OH
CH3CH2OH
��1��ȩ�����ʻ�(2�֣���1��) �ӳɷ�Ӧ(2��)
��2��  (2��)
(2��)
��3�� (2��)
(2��)
��4�� (�����𰸾���)(2��)
(�����𰸾���)(2��)
��5�� (5��)
(5��)
(ȫ����ȷ�ĵ�5�֣����д���ÿ��һ����ȷ�ĸ�1�֣����IJ��ø����������ֱ�ӵ�Ŀ��������)
���������������1��������F �к��������ŵġ�CHO��ȩ����>C=O���ʻ�����B����C�ĵı�����ɱ�����Ԫ�����Ǽӳɷ�Ӧ��
�к��������ŵġ�CHO��ȩ����>C=O���ʻ�����B����C�ĵı�����ɱ�����Ԫ�����Ǽӳɷ�Ӧ��
��2��д��������C�����ᷴӦ�������Ļ�ѧ����ʽ ��
��
��3�� B��(CH3)2C=CH2�뱽�Ӷ�λH�ļӳɷ�Ӧ���ṹ��ʽ ��
��
���㣺�л���ѧ������


 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����15�֣�
ij�о�С��Ϊ��̽��һ����������X����������Ԫ�أ�����ɺ����ʣ���Ʋ��������ʵ�飺
��ȡ10.80 g X�ڶ��������м�������ȫ�ֽ⣬�õ�6.40 g����1����ش��������⣺
��1��������ɫ����1�н���Ԫ�ص�ԭ�ӽṹʾ��ͼ_______ ��
д������ĵ���ʽ________ ��
��2��X�Ļ�ѧʽ��____ ���ڶ��������м���X����ȫ�ֽ�Ļ�ѧ����ʽΪ_______________ ��
��3����ɫ����2�ڿ����б�ɺ��ɫ������ԭ����________________ ��
���û�ѧ����ʽ��ʾ����
��4��һ�������£�����������1��ij�ֳɷֿ��ܷ���������ԭ��Ӧ��д��һ�����ܵĻ�ѧ��Ӧ����ʽ____________________________________________________________��
�����ʵ�鷽����֤�÷�Ӧ�IJ���_________________________________________
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��14�֣�п�̷ϵ�ؿ�������������п��̼���̣��乤�����̷�ΪԤ����������п�����ߡ�̼���������������֡���������̼���̵Ĺ����������£�
��ش��������⣺
��1��п�̷ϵ�صĻ��չ����У���ѭ�������Ƕȿ��ǣ�������������Դ������________��
��2��ԭ���̷۴�Ʒ����Ҫ�ɷ�ΪMnO2��̼������ʱһ����MnO2����ԭ����һ��
�ֱ�̼��ԭ��MnO2����ԭΪһ�����̡���֪��
д��C(s)��MnO2(s)����ΪCO(g)���Ȼ�ѧ����ʽ��________��
��3��50-55 ʱ����MnSO4��ĸҺ�м�������NH4HCO3����Ӧ�Ļ�ѧ����ʽΪMnSO4��
ʱ����MnSO4��ĸҺ�м�������NH4HCO3����Ӧ�Ļ�ѧ����ʽΪMnSO4��
��4����֪�������ӳ���ʱ��pH��Χ��Fe3+��2.7~3.7��Mn2+��8.6~10.1��Fe2+��7.6~9.6��������г�ȥ�ķ������������������¼���_________,��Fe2������ΪFe3����Ȼ���ٽ�pH����_______��ʹFe3��������ȫ��
��5����ƷMnCO3�����������������Һ��������Һ�ɵö������̣�д�������ĵ缫��Ӧʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣��ܻ����ֳ����ܼ�����һ�����Ӳ��ϵ������Ի�ʹ����Һ�������Ӽ�����������ʳƷ���������ҵ��
DEHP��C24H38O4�������ܼ���һ�֣���ͨ�����������Ʊ�������A����������6��̼ԭ�ӣ�D�ǶԶ��ױ���һ��ͬ���칹�壬E�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӡ�

��1��3���������ijͬ���칹�������ֻ����1�ֲ�ͬ��ѧ��������ԭ�ӣ���ϵͳ����������Ϊ ��
��2��д��B ��C�Ļ�ѧ��Ӧ����ʽ����ע����Ӧ�����ͷ�Ӧ���͡�
B ��C�� ����Ӧ���ͣ� ��
��3��E�Ľṹ��ʽ�� ��DEHP�Ľṹ��ʽ�� ��
��4��F��E��һ��ͬ���칹�壬��������������
a.�DZ�����λ��ȡ��� b.��FeCl3��Һ����ɫ�� c.����̼��������Һ��Ӧ��
д��F�����й����ŵ�����Ϊ_________________________��
��5��G��E�γɵĻ���ֻҪ���ʵ���һ�������۶��߱�����Σ�ȼ�պ�����һ��ֵ����������������Է���������С��G�ķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(15��)�л���A��H��������������֮���ת����ϵ���¡���֪��B��FeCl3��Һ�������ɫ�仯��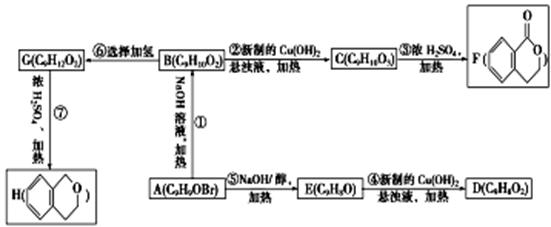
�ش��������⣺
��1��B�Ľṹ��ʽΪ ��
��2���١���������ȡ����Ӧ���� (�����)��
��3��д��һ�ֿɼ���D��F���Լ��� ��
��4��д����Ӧ�ߵĻ�ѧ����ʽ�� ��
��5��д��ͬʱ��������������C��ͬ���칹��Ľṹ��ʽ�� ��
�ٻ�������1,3,5-��ȡ����
�ڱ����ϵ�����ȡ�����зֱ��д��ǻ������ǻ���ȩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����ѧ����ѡ��5���л���ѧ��������15�֣�
�ϳ����������Ȼ�������Ҫ����֮һ����ˮ������ij�����F�ĺϳ�·�ߣ�����ͼ��ʾ����ͼ�е�R��R�䣬R�������������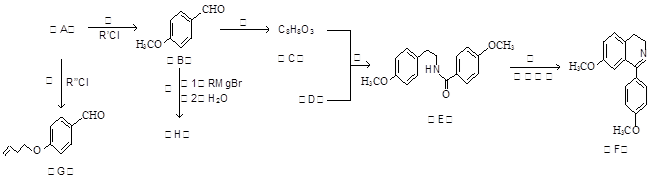
��֪������Ϣ��
��AΪ�����廯���ֻ��C��H��O����Ԫ�أ�1molA��ȫȼ��������7.5molO2��
�ڷ����к��С� ���ṹ�����ʿɷ������з�Ӧ��
���ṹ�����ʿɷ������з�Ӧ��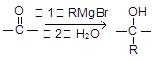
�������ǻ�������ͬһ��̼ԭ����ʱ�����ȶ���������ˮ��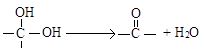
�ܺϳ�·���Т�͢���Ӧԭ����ͬ����͢�����ˮ���ɣ�
�ش��������⡣
��1����Ӧ���е�R��Cl��ϵͳ����Ϊ ��B�й����ŵ�����Ϊ ��
��2������������ȷ���� ��
a����������Cu(OH)2���Լ���A��B��C��������
b��D��PPA�� ����Ϊͬ���칹��
����Ϊͬ���칹��
c��E��G���ɷ����ӳɷ�Ӧ��ˮ�ⷴӦ
d��F�ķ���ʽΪC17H17NO2
��3��ij������ṹ��ʽΪ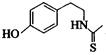 ������һ��������Ҳ�ܷ������Ƣ��Ļ�����Ӧ��д���û������������Ӧ�Ļ�ѧ����ʽ ��
������һ��������Ҳ�ܷ������Ƣ��Ļ�����Ӧ��д���û������������Ӧ�Ļ�ѧ����ʽ ��
��4����H��G��Ϊͬ���칹�壬��H�ɷ�����ȥ��Ӧ��д������Ҫ���H�ṹ��ʽ����дһ�֣�
��
��5����������֪ʶ����������Ϣ�����������X��CH3MgBrΪԭ���Ʊ�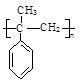 �ĺϳ�·������ͼ��
�ĺϳ�·������ͼ��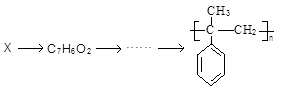
ע����X��B��Ϊͬ���칹�壬X�ĺ˴Ź���������4��壬�ҷ����֮��Ϊ3:2:2:1.
�����Լ����ã��л����ýṹ��ʽ��ʾ���ϳ�·������ͼʾ�����£�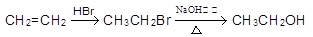
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣���֪AΪij�־ۼ���ϩ������ά��M���ĵ��壬��ת����ϵ���£�
�ش��������⣺
��1��B�й����ŵĽṹ��ʽΪ ��C������_____________________��ϵͳ������������
��2����ӦA��M�ķ�Ӧ����Ϊ ��M�Ľṹ��ʽΪ ��
��3���ٷ�ӦC��D�Ļ�ѧ����ʽΪ ��
��F��������Һ��Ӧ�����ӷ���ʽΪ ��
��4������E������˵����ȷ���� ����д��ţ���
�ٷ���������̼ԭ�Ӷ���ͬһƽ���� ������H2��Ӧ
������NaOH����Һ��Ӧ ������HBr��Ӧ
��5��д����������������A��ͬ���칹��Ľṹ��ʽ�� ��
����A������ͬ�Ĺ����� ��ˮ�����֮һ��ʽ��Ϊ108����FeCl3��Һ����ɫ
�ۺ˴Ź���������5�ַ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣�������Īɳ���֣�F����һ����ʹҩ�����ĺϳ�·�����£����е�THF���йط�Ӧ�Ĵ�������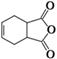
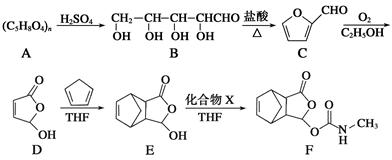
��1��������C�еĺ��������ŵ�����Ϊ �� ��������E�ķ����к��е�����̼ԭ����Ϊ ��1 mol F������ mol NaOH��Ӧ��
��2��������C������������ͭ������Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��д��ͬʱ��������������E��һ��ͬ���칹��Ľṹ��ʽ�� ��
���ӵĺ˴Ź�������ͼ��1H�˴Ź�����ͼ������4���壻���ܷ���������Ӧ��ˮ�ⷴӦ��������FeCl3��Һ������ɫ��Ӧ��������ˮ��Ӧ��
��4����֪E��X��FΪ�ӳɷ�Ӧ������X�Ľṹ��ʽΪ ��
��5����֪�� ������G���ṹ������ͼ��ʾ���Ǻϳɿ�����ҩ������Τ���м��塣��д����OHCCH(Br)CH2CHO
������G���ṹ������ͼ��ʾ���Ǻϳɿ�����ҩ������Τ���м��塣��д����OHCCH(Br)CH2CHO
��1,3������ϩΪԭ���Ʊ�G�ĺϳ�·������ͼ�����Լ����ã���
�ϳ�·������ͼʾ�����£�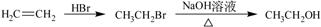
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
������Ӵ����ڶ����͡������͵��У����������ƿ���ܰ���㾫�Լ����춡��Ӻ������صȣ�Ҳ����ɱ����ͷ�������������ӽṹ��ʽ��ͼ��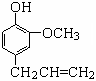
��1��������ӵķ���ʽΪ
��2���������ʲ��ܸ�������ӷ�Ӧ����
| A��NaOH��Һ | B��NaHCO3��Һ | C��Na2CO3��Һ | D����ˮ |
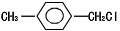 ���Ҵ�Ϊԭ���Ʊ�������ӵ�һ��ͬ���칹�壺��-�������������ĺϳ�·�ߣ����Լ���ѡ����
���Ҵ�Ϊԭ���Ʊ�������ӵ�һ��ͬ���칹�壺��-�������������ĺϳ�·�ߣ����Լ���ѡ����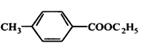
 B����
B���� Ŀ����
Ŀ�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com