
(1)��ˮ�ԣ�Ũ����������տ����е�_________�����ڹ����е�_________���ᾧˮ�����е�_________��������һ����ʵ���ҳ���Ũ������_________��
(2)��ˮ�ԣ�ָ�л��������е�_________Ԫ�ذ�_________����������������ϳ�_________��
(3)ǿ�����ԣ�����ʱ��C+2H2SO4(Ũ)![]() __________________���ڳ����£�����_________��ŨH2SO4�����γ�_________����ֹ��������Ľ�һ����Ӧ���������֮Ϊ_________��
__________________���ڳ����£�����_________��ŨH2SO4�����γ�_________����ֹ��������Ľ�һ����Ӧ���������֮Ϊ_________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
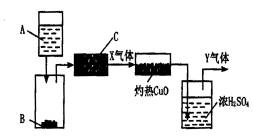
ͼ��A��Һ�壬B��C�ǹ��塣A��B��Ӧ����������徭��C���õ�������X��Xͨ������CuO��CuOת��ΪCu��������ͨ��ŨH2SO4�����õ�������Y��Y�ǶԻ���û����Ⱦ�����壬�����ŷ�Ҳû��Σ�ա�
Ϊ��������ʵ�飬��������Լ���Χ��ѡ����ʵ�A��B��C��Ũ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢп����ŨNaOH��Һ������ʯ����ʯ�ҡ�NH4CI���塣

��1��A��________��B��________��C��________��X��________��
��2��д��A��B��X��CuO��Ӧ�Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�043
��ͼ��һ��ʵ��ʾ��ͼ�������ʾ�йص���������ͷ��ʾҺ������������
ͼ��A��Һ�壬B��C�ǹ��塣A��B��Ӧ����������徭��C���õ�������X��Xͨ������CuO��CuOת��ΪCu��������ͨ��ŨH2SO4�����õ�������Y��Y�ǶԻ���û����Ⱦ�����壬�����ŷ�Ҳû��Σ�ա�
Ϊ��������ʵ�飬��������Լ���Χ��ѡ����ʵ�A��B��C��Ũ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢп����ŨNaOH��Һ������ʯ����ʯ�ҡ�NH4CI���塣
��1��A��________��B��________��C��________��X��________��
��2��д��A��B��X��CuO��Ӧ�Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��־��ϵ�б���һѵ����ѧ�ս̰� �ս̰� ���ͣ�022
��H2SO4��________Һ�壬�е�________��________�ӷ���________����ˮ����ˮ________���ܽ�ʱ�ų�________��
(1)��ˮ�ԣ�Ũ����������տ����е�________�����ڹ����е�________���ᾧˮ�����е�________��������һ����ʵ���ҳ���Ũ������________��
(2)��ˮ�ԣ�ָ�л��������е�________Ԫ�ذ�________����������������ϳ�________��
(3)ǿ�����ԣ�����ʱ��C��2H2SO4(Ũ)![]() ________���ڳ����£�����________��ŨH2SO4�����γ�________����ֹ��������Ľ�һ����Ӧ���������֮Ϊ________��
________���ڳ����£�����________��ŨH2SO4�����γ�________����ֹ��������Ľ�һ����Ӧ���������֮Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��ϡHClO4��Һ��ŨH2SO4��Ϻ��ѹ����
B.��ϡHClO4ע��ŨH2SO4���ٽ����������¶ȿ�����130 ��
C.�����ý�������Ƥ�����Ӹ�����
D.���������Ľ���Ҫ���������ã����ܿ��ܷ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com