��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO
2�ĺ�������Ч�ؿ�������CO
2�������˸������ձ����ӣ�
��1����ѧ���Խ��ܼ���Ϊ�����ĵ�̼����������أ���������Υ����չ��̼���õ���
B
B
������ţ�
A�����ԭ�������ʣ���չ��ɫ��ѧ
B��������չ������ҵ�������������������
C���ƹ�ú��������Һ���������ṩ����Чȼ��
D����������̫���ܡ����ܡ����ܡ����ܵ���Դ
��2�������˺���������̬ϵͳ�У�����Ҫ������ȥCO
2����Ҫ���ṩ�����O
2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO
2�T2CO+O
2��CO������ȼ�ϣ���֪�÷�Ӧ��������ӦʽΪ��4OH
--4e
-��O
2��+2H
2O����������ӦʽΪ
2CO2+4e-+2H2O=2CO+4OH-��
2CO2+4e-+2H2O=2CO+4OH-��
��
��3��CO
2��ת�����л���ʵ��̼ѭ���������ΪlL���ܱ������У�����1mol CO
2��3mol H
2��һ�������·�����Ӧ��
CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g����H=-49.0kJ?mol
-1��
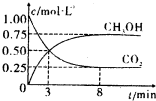
���CO
2��CH
2OH��g����Ũ����ʱ��仯��ͼ��ʾ��
�ٴ�3min��8min��v��H
2��=
0.15mol/��L?min����
0.15mol/��L?min����
��
����˵��������Ӧ�ﵽƽ��״̬����
D
D
�����ţ���
A����Ӧ��CO
2��CH
2OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B�����������ܶȲ���ʱ�ŵı仯���仯
C����λʱ����ÿ����3mol H
2��ͬʱ����1mol H
2O
D��CO
2����������ڻ�������б��ֲ���
��4����֪��
��C��s��+O
2��g���TCO
2��g������H
1=-437.3kJ?mol
-1��2H
2��g��+O
2��g���T2H
2O��g������H
2=-571.6kJ?mol
-1��2CO��g��+O
2��g���T2CO
2��g������H
3=-566.0kJ?mol
-1��C��s��+H
2O��g���TCO��g��+H
2��g������H=
+131.5kJ?mol-1
+131.5kJ?mol-1
��
��5��ij���ױ�������30�桢30MPa�����£���Fe��COCl
2��������CO
2��H
2��Ӧ���ɶ�������飮�ٶ���һ�����г���һ������CO
2��H
2�������������CO
2��H
2��ת���ʾ�Ϊ100%������ֻ�ж�������飬
=a����a��ȡֵ��ΧΪ
3.2��a��3.25
3.2��a��3.25
��

 ��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�