

| ��� | ��ѧʽ |
| �� | |
| �� | |
| �� | |
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����



| ѡ�õ�����������ĸ�� | ������Լ� | ���� |
| | | ��Ӧ�����������壩 |
| C | | |
| | ����ͭ | ʹ����������ͭ��Ӧ |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽�� �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����


 I����֤
I����֤ �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��| A��Ba(HCO3)2��Һ | B�������� | C����ˮ | D��Ʒ����Һ |
 �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)�� �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
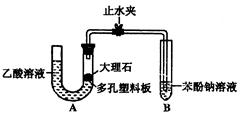
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �������ϣ���Cu2O���ڼ�������� ���ڿ���������Cu2O����CuO����Cu2O�������������ܷ������з�Ӧ��Cu2O��2H+��Cu��Cu2+��H2O��
�������ϣ���Cu2O���ڼ�������� ���ڿ���������Cu2O����CuO����Cu2O�������������ܷ������з�Ӧ��Cu2O��2H+��Cu��Cu2+��H2O�� �鷽����
�鷽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
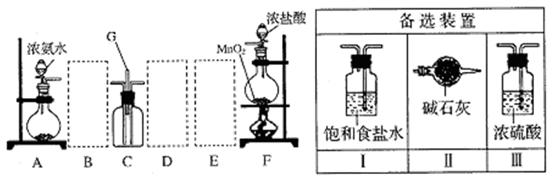
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 (����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������
(����ֵ)��ʵ�顣�ȳ�����Ӧ������ͭ������m(CuO)����Ӧ��ȫ��ⶨ������ˮ������ m(H20)���ɴ˼���
m(H20)���ɴ˼��� ��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��
��Ϊ�ˣ��ṩ��ʵ���������Լ�����(������Ҫ���ظ�ѡ�ã������NH4C1��Ca(OH)2�������Բ���ʹCuO��ȫ��ԭ�İ���)��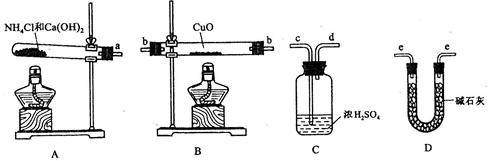
 ����_______________ (�����)��
����_______________ (�����)�� ����
���� _____��_______________�ﵽʵ��Ŀ�ġ�
_____��_______________�ﵽʵ��Ŀ�ġ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �۾��г�ǿ�Ĵ����ܣ����������ܶȴż�¼�Ľ����Լ���Ч�����ȡ��ڲ�ͬ�¶��£�
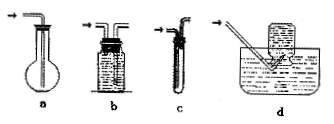
�۾��г�ǿ�Ĵ����ܣ����������ܶȴż�¼�Ľ����Լ���Ч�����ȡ��ڲ�ͬ�¶��£� ����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570��ʱ������FeO������570��ʱ������Fe3O4��ijУ��ѧС��ѧ��������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�飬��̽���������ɷ֣�ͼ�мгּ�β������װ�þ�����ȥ����
����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570��ʱ������FeO������570��ʱ������Fe3O4��ijУ��ѧС��ѧ��������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�飬��̽���������ɷ֣�ͼ�мгּ�β������װ�þ�����ȥ����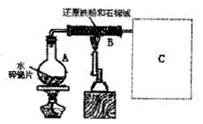 |
 |
| ʵ�鲽�� | ʵ����� | ʵ������ |
| �� | ����Ӧ��õ���ɫ��ĩX���ٶ�Ϊ���ȵģ���ȡ������������һ�Թ��У������������ᣬ�� | ��ɫ��ĩȫ���ܽ⣬��Һ�� �����������ݲ��� |
| �� | ��ʵ����еõ�����Һ�Ӽ���KSCN��Һ���� | ��Һ����Ѫ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �� �� ��
�� �� ��| A������ˮ�Ҵ��ӵ�ˮ����ȡ���ʵ� |
B��ϡ��Ũ ����ʱ��Ӧ��Ũ���Ỻ��ע��ʢ��ˮ����Ͳ�� ����ʱ��Ӧ��Ũ���Ỻ��ע��ʢ��ˮ����Ͳ�� |
| C�����ƣ�FeCl3��Һʱ������Һ�м�������Fe��ϡ���� |
| D���ᴿ��������������Ҵ������ȼ���ʯ�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com