
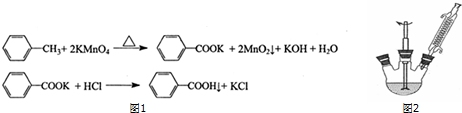
 �л���ķ�Ӧ�������渱��Ӧ�����������Ҫ�����ᴿ����һ��ˮ���㾫�ĺϳɲ������£�
�л���ķ�Ӧ�������渱��Ӧ�����������Ҫ�����ᴿ����һ��ˮ���㾫�ĺϳɲ������£�| ������ | ������ | ������ | ���������� | ������ |
| �ܶ�/��g/mL�� | 0.810 | 1.049 | 0.882 | 0.7689 |
| �е�/�� | 117.8 | 118.1 | 126.1 | 143 |
| ��ˮ�е��ܽ��� | ���� | ���� | ���� | ���� |
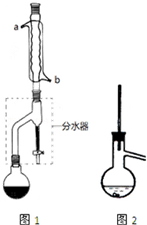
���� ��1�����������������ŨH2SO4���������·���������Ӧ����������������ˮ��
��2�������ܲ�ȡ����ԭ��ͨ������ˮ����������ȴ��
��3�������������к������ᡢ���������������ʣ�����������������������ˮ�����������������ܣ�������뱥��̼���Ʒ�Ӧ���Դ˽����⣮
��4����������ˮ����þ�����Ӧ���˳�ȥ���������Ӧ�з�������Ӧ�õ������ѣ������������ܣ�����������������ˮ���Ʊ��������к��������ѣ�
��5�������������ķе���126.1�棬ѡ��е��Ըߵ�Һ����ȣ�
��6����������ˮ�����������ݷ���ʽ����μӷ�Ӧ�������IJ�������������ת����=$\frac{�μӷ�Ӧ������}{Ͷ���������}$��100%��
��� �⣺��1�����������������ŨH2SO4���������·���������Ӧ����������������ˮ����ѧ����ʽΪCH3CH2CH2CH2OH+CH3COOH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH2CH2CH3+H2O��
�ʴ�Ϊ��CH3CH2CH2CH2OH+CH3COOH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH2CH2CH3+H2O��
��2�������ܲ�ȡ����ԭ��ͨ������ˮ��������ˮӦ��b�ܿ�ͨ�룬��a������������������ȴ��
�ʴ�Ϊ��b��
��3�������������к������ᡢ���������������ʣ����������������ڱ���̼������Һ�������������������ܣ��������̼���Ʒ�Ӧ�������գ����ñ���̼������Һ��ȥ���������������ᡢ���������������ʣ��ʴ�Ϊ�����ᡢ���ᡢ��������
��4����������ˮ����þ�����Ӧ���˳�ȥ�������������������������ˮ������ˮϴ��10%̼������Һϴʱ���Ѿ�����ȥ�������������ᶡ�����ܣ�ˮϴ��10%̼������Һϴʱ���ܳ�ȥ����������ʱ���������ӷ��������ᶡ����������������������Ϊ�����ѣ�
�ʴ�Ϊ�����ˣ������ѣ�
��5�������������ķе���126.1�棬ѡ��е��Ըߵ�Һ����ȣ����Կ����ڸ��ͺ�ʯ�����м��ȣ�ˮ�ķе�Ϊ100�棬�����¶�̫�ͣ���ɰ�Ӽ����¶�̫�߲����ƣ�
��ѡ��bd��
��6����Ӧ���������ų���ˮΪ6.98mL��ˮ���ܶ�Ϊ1g•mL-1������Ӧ���ɵ�ˮΪ6.98mL-5.00mL=1.98mL����1.98g����μӷ�Ӧ��������Ϊx��
CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O
74 18
x 1.98g
��x=$\frac{74��1.98g}{18g}$=8.14g��
������������������ת����=$\frac{�μӷ�Ӧ������}{Ͷ���������}$��100%=$\frac{8.14g}{9.3g}$��100%=88%��
�ʴ�Ϊ��88%��
���� ���⿼���л����Ʊ�ʵ�飬�漰ʵ�������ʵ��ԭ�������ʵ����ʵ�Ӧ�á������ᴿ���йط���ʽ�ļ���ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ��̽�����������ݴ���������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2CO3?2H++CO32- | B�� | NaHSO4=Na ++HSO4- | ||
| C�� | NaHCO3=Na++H++CO32- | D�� | NH4Cl=NH4++Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
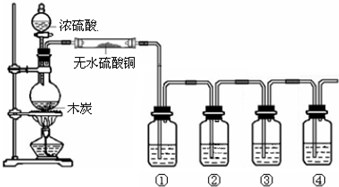
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ���� | ��Է������� | �ܶ�/��g•mL-1�� | �е�/�� | ˮ���ܽ��� |
| CHCl3 | 119.5 | 1.50 | 61.3 | ���� |
| CCl4 | 154 | 1.59 | 76.7 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ���� | ��Է������� | ��״ | �۵�/�� | �ܽ�� | |
| ˮ | �Ҵ� | ||||
| �ױ� | 92 | ��ɫҺ����ϩ�ӷ� | -95 | ���� | ���� |
| ������ | 122 | ��ɫƬ״����״���� | 122.4��100�������� | 25��0.35g 80��2.7g | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��BA4�Ľṹʽ
��BA4�Ľṹʽ ��
�� ��
���鿴�𰸺ͽ���>>
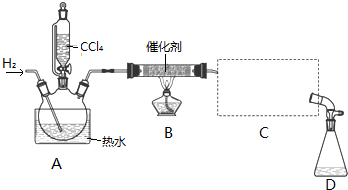
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
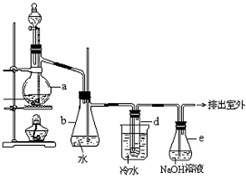 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ������ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4oC���ܶ�Ϊ1.43g•ml-1��
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ������ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4oC���ܶ�Ϊ1.43g•ml-1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S2-���ӵĽṹʾ��ͼ��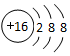 | B�� | �������Ļ�ѧʽ��AlSO4 | ||
| C�� | H2O2��O�Ļ��ϼ�Ϊ-2�� | D�� | ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ��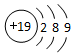 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
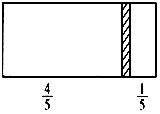 һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���1molN2���ұ߳���һ������COʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ���ǣ�������
һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���1molN2���ұ߳���һ������COʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ���ǣ�������| A�� | �ұ�����߷�����֮��Ϊ4��1 | |
| B�� | �Ҳ�CO������Ϊ5.6 g | |
| C�� | �Ҳ������ܶ�����ͬ�����������ܶȵ�14�� | |
| D�� | ���ı��ұ�CO�ij�������ʹ���崦���������м䣬�����¶Ȳ��䣬��Ӧ����0.2molCO |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com