
��10�֣�����֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ��ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ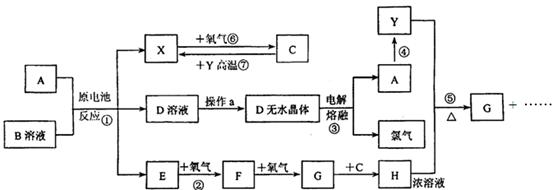
����д���пհף���1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ��
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ ��
��1��2Mg+CO2=2MgO+C��2�֣���2����D��Һ��HCl���������ɣ�2�֣�
��3��4NH3+5O2 4NO+6H2O��2�֣�
4NO+6H2O��2�֣�
��4��C+4HNO3��Ũ�� CO2��+4NO2��+2H2O��2�֣�
CO2��+4NO2��+2H2O��2�֣�
��5��2NH +2e����2NH3��+H2����2�֣�
+2e����2NH3��+H2����2�֣�
�������������������Ŀ��Ϣ��֪CΪҺ�壬��CΪˮ��
Ϊ��������������������ˮ�ķ�Ӧ������֪EΪ������HΪ���ᣬ���ݷ�Ӧ�ޣ�XΪH2,��Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������,����֪AΪþ��YΪ̼��E-HΪ��ҵ������Ĺ��̣�BΪ�Ȼ����Һ��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ2NH +2e����2NH3��+H2��
+2e����2NH3��+H2��
���㣺�����˳�������þ�����ʡ���ѧ����ʽ����д��ԭ��صĵ缫��Ӧʽ��
�������������ڳ��������ƶ��⣬������Ŀ��Ϣ�ҳ�����CΪˮ���ٸ��ݳ��������������������Ƴ�EΪ���������ݳ������ʵ�������Ӧ�ƶ���������Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
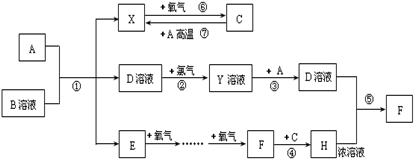
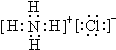
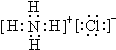
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ����ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
����д���пհף�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ
______________________________________________________________________��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ____________________________��
��3��E�ĵ���ʽΪ_________________________________��
��4����Ӧ�ݵĻ�ѧ����ʽΪ_______________________________________________��
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ___________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮ��һ�и������Ľο��Ի�ѧ�Ծ� ���ͣ������
(10��)��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ��
��ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
����д���пհף�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����������
��4����Ӧ�ݵĻ�ѧ����Ϊ ����������
��5��ԭ��ط�Ӧ���������ĵ缫��ӦΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com