
(10��)��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ��
��ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
����д���пհף�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����������
��4����Ӧ�ݵĻ�ѧ����Ϊ ����������
��5��ԭ��ط�Ӧ���������ĵ缫��ӦΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 400 | 500 | 830 | 1000 |
| ƽ�ⳣ��K | 10 | 9 | 1 | 0.6 |
| [H2]?[CO2] |
| [CO]?[H2O] |
| [H2]?[CO2] |
| [CO]?[H2O] |
| A | B | C | D | |
| n��CO2�� | 3 | 1 | 0 | 1 |
| n��H2�� | 2 | 1 | 0 | 1 |
| n��CO�� | 1 | 2 | 3 | 0.5 |
| n��H2O�� | 5 | 2 | 3 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
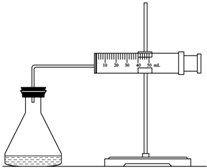 ʵ������H2O2�ֽⷴӦ��ȡ����ʱ������������Լӿ췴Ӧ���ʣ�ij�о���ѧϰС��Ϊ�о�����FeCl3������O2�������ʵ�Ӱ�죬�������������ʵ�鷽�������±������������������Լ���һ�������Ϻ���з�Ӧ��
ʵ������H2O2�ֽⷴӦ��ȡ����ʱ������������Լӿ췴Ӧ���ʣ�ij�о���ѧϰС��Ϊ�о�����FeCl3������O2�������ʵ�Ӱ�죬�������������ʵ�鷽�������±������������������Լ���һ�������Ϻ���з�Ӧ��| ʵ���� �Լ� |
A | B | C |
| 10% H2O2/mL | 20.0 | V1 | V2 |
| 2mol/L FeCl3/mL | 0 | 5.0 | 10.0 |
| H2O/mL | V3 | V4 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ������
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��
(1) ��O?30Сʱ�ڣ�CH4��ƽ���������� ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g) CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3) �÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ���� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g)  CH3OH (g), ��H<0�ϳɼ״�����230��C?270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
CH3OH (g), ��H<0�ϳɼ״�����230��C?270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
��2700C��ʵ��������Ӧ��������_________ (����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________ (����ĸ����
A. 1 ?1.5 B. 2. 5?3 C. 3. 5?4. 5
(5) ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ��ͳ����ѧ�Ծ� ���ͣ������
(16��)������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�
��1������ͼװ���У��۲쵽ͼ1װ��ͭ�缫�ϲ�����������ɫ���ݣ���ͼ 2װ����ͭ�缫����������������缫�ϲ���������ɫ���塣��ͼ 1 ֪�������Ļ�Ա�ͭ_____(��ǿ����)��ͼ 2װ���и��缫�ĵ缫��Ӧʽ

��2��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬ CrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________________��
(3)����ƽ�⣺2CrO42������ɫ��+2H+ Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O
����ƽ����ϵ��pH=2������Һ�� ɫ.
����˵���ڢٲ���Ӧ��ƽ��״̬���� ��
a��Cr2O72����CrO42����Ũ����ͬ b��2v (Cr2O72��) =v (CrO42��) c����Һ����ɫ����
��4��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ�����6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH��������� Fe(OH)3 ��Cr(OH)3������ȥ[��֪ KspFe(OH)3��4.0��10-38��KspCr(OH)3��6.0��10-31]��
�ٵ������� NaCl ��������__________________________��
����֪�������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L1������Һ��c(Cr3+)Ϊ____ mol��L-1��
��5��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ��

�ӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com