
����Ŀ��ij�о���ѧϰС���������ͼװ����ȡ����֤SO2�����ʡ�

��ش�
(1)д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ_______________________________��
(2)������NaOH ��Һ��������_______________________________��д���÷�Ӧ���ӷ���ʽ_______________________________��
(3)Ϊ����֤SO2�����������ϴ��ƿ���п�ѡ����Լ���_____��
A.���Ը��������Һ B.��ɫ��̪��Һ C.����ʯ��ˮ D.��ˮ
(4)����˵����ȷ����_____��
A.ʵ�鿪ʼʱ��ֻ���Һ©��������������ʹҺ��˳������
B.����װ���м����Լ�(ҩƷ)���ٽ��������Լ��
C.ʵ�鿪ʼ��ϴ��ƿ�ٺ͢�����Һ����ɫ�����߾���֤��SO2����Ư����
D.ʵ�鿪ʼ��ϴ��ƿ���пɹ۲쵽��ɫ�����������������˵��SO2���л�ԭ��
(5)���������ŷŵ������У����γ����꣬���������������ú�pH_____(����С��)������Ϊ�������������;���ɲ�ȡ�Ĵ�ʩ��_____��
������ú��ȼ�ϣ� �ڰѹ����̴���ߣ� ��ȼ������ �������ữ�������м�ʯ�ң��ݿ����µ���Դ��
A.�ڢ� B.�ڢۢ� C.�٢ۢ� D.�٢ۢܢ�
���𰸡�Cu+2H2SO4 ![]() CuSO4+SO2��+H2O ���ն���Ķ�������ֹ��Ⱦ���� SO2+2OH-=SO32- +H2O C D ��С C
CuSO4+SO2��+H2O ���ն���Ķ�������ֹ��Ⱦ���� SO2+2OH-=SO32- +H2O C D ��С C
��������
Ũ�����Cu�ڼ��������·�Ӧ���ɶ���������Ʒ����Һ�����������Ҫ�������������������������Ҫͨ�����Ե�����֤�����������ˮ��Ӧ�����ἴ�ɣ��ܶ��������ܱ�����������������������ӣ���������Ӻͱ����ӷ�Ӧ���ɰ�ɫ���ᱵ���������������ж�����ֱ���ſգ������ü�Һ���ն�������β�����ݴ˷������
(1)װ��A��Ũ������ͭ��Ӧ���ɶ�������Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4 ![]() CuSO4+SO2��+H2O���ʴ�Ϊ��Cu+2H2SO4
CuSO4+SO2��+H2O���ʴ�Ϊ��Cu+2H2SO4 ![]() CuSO4+SO2��+H2O��
CuSO4+SO2��+H2O��
(2)���������ж�������ֱ���ŷţ���Ϊ����������������������Կ����ü�Һ���ն�������β����������NaOH��Һ�����������ն�������β������ֹ��Ⱦ��������Ӧ���ӷ���ʽΪSO2+2OH-=SO32- +H2O���ʴ�Ϊ�����ն�������β������ֹ��Ⱦ������SO2+2OH-=SO32- +H2O��
(3)Ϊ����֤SO2���������������ͨ�������������ˮ��Һ�����Լ��ɣ����������ˮ��Ӧ���������ᣬ���������������Ӷ�������Һ�����ԡ�A.���Ը��������Һ��ɫ�����ֶ�������Ļ�ԭ�ԣ���ѡ��B.��ɫ��̪��Һ�����ɫ����ѡ��C.����ʯ��ˮ�������ᷴӦ���ɱ�ɫ�������ܹ�˵�������������ԣ�ѡ��D.��ˮ��ɫ�����ֶ�������Ļ�ԭ�ԣ���ѡ���ʴ�Ϊ��C��
(4)A��ʵ�鿪ʼʱ��ֻ���Һ©������������Һ©����ƿ�������������ѹ���ڷ�Һ©������ѹ��Һ�岻��˳�����£���A����B��Ӧ���ȼ���װ�������ԣ�Ȼ����װ���м����Լ�(ҩƷ)����B����C����������ʹƷ����Һ��ɫ���ֶ��������Ư���ԣ����ܱ���������ԭ����HBr����ɫ������ˮ��ɫ���ֶ�������ԭ�ԣ�����ʵ�鿪ʼ��ϴ��ƿ�ٺ͢���Һ����ɫ��ǰ������Ư���ԡ��������ֻ�ԭ�ԣ���C����D��������������������������������ӣ���������Ӻͱ����ӷ�Ӧ�������ᱵ����������ʵ�鿪ʼ��ϴ��ƿ���пɹ۲쵽��ɫ�����������������˵��SO2���л�ԭ�ԣ���D��ȷ���ʴ�Ϊ��D��
(3)���������ŷŵ������У����γ����꣬���������ױ������������ᣬ���ú������������ǿ��pH��С����ԭú�к�����Ԫ�أ�����ԭú��ȼ�ϣ����ܼ��ٶ���������ŷţ����Կ��Լ�������IJ�������ȷ���ڰѹ������̴���ߣ������α������ܼ�������IJ���������ȼ�������Լ��ٶ���������ŷţ����Լ�������IJ�������ȷ���������ữ�������м�ʯ�ҵȴ�ʩ�����ܼ���������γɣ����ݿ����µ������Դ���ܼ��ٶ���������ŷţ����Լ�������IJ�������ȷ����ѡC���ʴ�Ϊ����С��C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
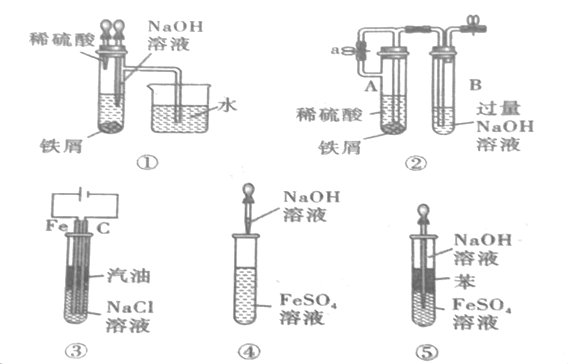
����Ŀ����ͼ��ʾ����װ�ÿ�������ȡ�۲�Fe(OH)2�ڿ����б�����������ɫ�仯��ʵ��ʱ����ʹ����м��6molL-1�����ᣬ�����Լ���ѡ����д���пհף�

��1��B��ʢ��һ������NaOH��Һ��A��ӦԤ�ȼ����ҩƷ��__��A�з�Ӧ�����ӷ���ʽ��__��
��2��ʵ�鿪ʼʱ�Ƚ�����a__(����رա�)��
��3����������Fe(OH)2�IJ������̣�__��
��4��ʵ����ϣ���b������������һ���ֿ�������ʱBƿ�з����ķ�ӦΪ__��
��5�����и�ͼʾ�У�___�ܽϳ�ʱ�俴��Fe(OH)2��ɫ������(�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Cl2�ķ�ӦΪ4HCl(Ũ)+MnO2![]() MnCl2+Cl2��+2H2O������˵���������( )
MnCl2+Cl2��+2H2O������˵���������( )
A. ��ԭ����HCl����������MnO2
B. ÿ����1 molCl2��ת�Ƶ��ӵ����ʵ���Ϊ2 mol
C. ÿ����1 molMnO2����ԭ�����õ�HCl����4mol
D. ���ɵ�Cl2�У�������һЩˮ�����⣬������HCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�����ײ��������������к���ֲ�����������ȫ�����֣���������������������Ŀ���Ԫ����ʹ����NH4NO3��KNO3��CaCl2��2H2O��MgSO4��7H2O����������Һ����Ԫ����Һ����ȱ����һ�ֱ���Ԫ�أ�Ϊ��������Ԫ�أ�Ӧ���ӵĻ������ǣ� ��

A.Ca��NO3��2B.KClC.KH2PO4D.K2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�Ļ�������������������������Ҫ�����á�
��1����֪��2CO(g)+O2(g)![]() 2CO2(g) ��H1=��566kJ��mol��1
2CO2(g) ��H1=��566kJ��mol��1
H2O(g)+CO(g)![]() H2(g)+CO2(g) ��H2=��41kJ��mol��1
H2(g)+CO2(g) ��H2=��41kJ��mol��1
CO(g)+2H2(g)![]() CH3OH(g) ��H3=��107kJ��mol��1
CH3OH(g) ��H3=��107kJ��mol��1
��CH3OH(g)+![]() O2(g)
O2(g) ![]() CO2(g)+2H2O(g) ��H=___kJ��mol��1
CO2(g)+2H2O(g) ��H=___kJ��mol��1
��2��T��ʱ�����ݻ���Ϊ2L��A��B�����ܱ������о�ͨ��4.0molCO2��6.8molH2��������Ӧ��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H=-50kJ��mol��1��A�����еķ�Ӧ�ں��¡����������½��У�B�����еķ�Ӧ�ں��¡���ѹ�����½��У����A������CO2��ת���� �� (CO2) ��ʱ��ı仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=-50kJ��mol��1��A�����еķ�Ӧ�ں��¡����������½��У�B�����еķ�Ӧ�ں��¡���ѹ�����½��У����A������CO2��ת���� �� (CO2) ��ʱ��ı仯��ͼ��ʾ��

����0~5min��A������v(CH3OH)=___�����¶���������Ӧ��ƽ�ⳣ��K=___(������λ��Ч����)��
�ڷ�Ӧ��ʼ��ƽ��Ĺ����У�A��B��������CO2���������ʵĴ�С��ϵΪv(A)___(����>����<������=��)v(B)��
�۷�Ӧ�����У����и���ָ���ܱ���A�����з�Ӧ��v��>v������___(����)
a.��ϵ�ڵ�ѹǿ����
b.�����ƽ����Է�����������
c.����H��H������Ŀ���γ�C��O����Ŀ��2��
d.v��(CO2)=v��(H2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������������̬������ʵ��������Ȼ�ĺ�г������
������������Ӿ�����ЧӦ����__________������ĸ����
a.ֲ������ b.ȼú��ů c.��������
�����з��Ρ���ɫ��Ⱦ������ȷ������_____________������ĸ����
a.ʹ�ÿɽ������� b.¶����շϾ����� c.ֱ������Ͼ�����
��Ϊ���������Ⱦ����������ѽ�ֹȼ���̻�������ֹȼ���̻����ı�ʶ��_____������ĸ����

��2������ʹ�û�ѧ֪ʶ��������ǵ�����������
ijƷ������ijɷ��и��͡�ɽ����ء������Ƶȡ�
������������ɷ��У����ڷ���������_______________��
�ڸ��͵Ľṹ��ʽΪ____________����֬ˮ������ɸ��ͺ�_____________��
�۷�����(NaF)���������е��ǻ������[Ca5(PO4)3OH]��Ӧ�����ɸ����ܵķ������[Ca5(PO4)3F]���Ӷ��ﵽ����ȣ�ݵ�Ŀ�ġ�д���÷�Ӧ�Ļ�ѧ����ʽ��____________________��
��3�����·�չ���ϼ������ƶ��������Ľ�����
��ʯīϩ������ͼ��������̫���ܵ�صĵ缫��������Ҫ������ʯīϩ��______________�ԡ�

�ڻ������̽����г��õ�ˮ�ࡢ�������ֲĵȡ�����ˮ��Ͳ������õ���ԭ����__________���ڸֲ������Ӹ�������Ԫ�ص�Ŀ����___________��
������ս������������SiC������Ϊ�������ϡ������½�̿��ʯӢ��Ӧ���Ƶ�SiC��ʯӢ�Ļ�ѧʽΪ________________�����·ֽ�Si(CH3)2Cl2Ҳ���Ƶ�SiC��ͬʱ������CH4��һ�ֳ����������壬д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��2015��11����C919��ɻ���װ���ߣ���־���ҹ������ɻ������Ƚ��������С�
�ٻ�����Ƥʹ�õ��ǵ�������﮺Ͻ���ϡ����в�������﮺Ͻ����ʵ���___������ĸ����
a���ܶȴ� b��Ӳ�ȴ� c������ʴ
�ڷɻ���̥��������Ʒ�����в��Ͽ������ϳ�����____������ĸ����
a��ʯӢɰ b��ʯ��ʯ c�������ϩ
�ۻ����Ƚ����ϲ��ϵ�ʹ���ʴ�12%���������ڸ��ϲ��ϵ���___������ĸ����
a���ѺϽ� b�������� c����ͨ�մ�
��2�������һ�ִ���ϲ����ʳ�ģ����е����ʡ�ά����A��ά����C����ά�ء����������Ǻͷḻ������п���Ƶ�Ԫ�أ����У�

����������������Ԫ�ص���____��
������ˮ����ά���ص���____������������а��������ǡ�___��
�۵����������������յ�ˮ�������_____��
��3����ѧ�ܰ������Ǹ��õ���ʶ����������
�ټ�������δϴ�����ɣ�����һ��ʱ�����ֺ��ɫ��ߣ�����Ҫ��ԭ������������____��ʴ��
�ڳ˳���ֹЯ����Ʒ�IJ��ֱ�ʶ��ͼ��ʾ����Ũ���ᡢʳ�Ρ����͡�ʯ��������Ʒ�У�����Я���ϳ�����_____��

�۹������ᣨ![]() ���ֽ�Ϊ�������������ϡ�ܳ����ڲ;ߡ���е��������д����������ֽ�Ļ�ѧ����ʽ______��ijθҩ����Ҫ�ɷ���AlbMgc��OH��mCO3������������θ����࣬д��������θ�ᷴӦ�Ļ�ѧ����ʽ_______��
���ֽ�Ϊ�������������ϡ�ܳ����ڲ;ߡ���е��������д����������ֽ�Ļ�ѧ����ʽ______��ijθҩ����Ҫ�ɷ���AlbMgc��OH��mCO3������������θ����࣬д��������θ�ᷴӦ�Ļ�ѧ����ʽ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����»Ὣ��2014��8�����Ͼ����С���»Ὠ����ʩʹ���˴������ܲ��ϣ�����������ɫ����������
��������´幤�̵ĸ��ȱ��²��Ͼ۰�������______������ĸ����
a���л��߷��Ӳ��� b�����ǽ������� c����������
�����������ǽ�ĸ��ϱ��²��ϲ������Ͻ����ߡ��й����Ͻ��������ȷ����___������ĸ����
a��ǿ��С b����ʴ c���ܶȴ�
����»�������˫�������˲���Ļǽ��ơ����첣������Ҫԭ��Ϊ���ʯ��ʯ��___������ĸ����
a�����ɰ b��ʯӢ c��ˮ����
��2����ͼΪijƷ�ƽ��ͱ�ǩ��һ���֡�

�ٰ�����̬���ĺ����ǽ�����������Ҫָ�꣬��Щ���������ɴ��е���ҪӪ������_____ˮ������ġ�
�������嵥�����ڷ���������______��
��С���е���ҪӪ��������_____��д������������ȫˮ��Ļ�ѧ��ʽ��______��
��3��A��B��C��������ȫ����ˮ����ƽ��pH�仯��ͼ��ʾ��
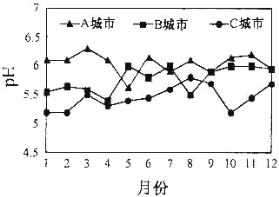
��������Σ�������ص���____���С�
�ڵ��������������γ���Ҫ������___������ȼú���̲���������Ĵ�ʩ��_________��
������β���к���NO2��NO��CO���к����塣д����NO2�γ�����������Ļ�ѧ����ʽ��______��������װβ������װ�ÿɽ�NO��COת��Ϊ�����壬д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش���������
��1���ҹ��涨������ˮ�����ŷŵ��������Ũ��Ϊ0.005 mg/L���������ӷ�ˮ�ɲ��û�ѧ���������Իش��������⣺
�������ӣ�Cd3(PO4)2�������ܽ�ƽ�ⳣ���ı���ʽKsp��_____________________��
����ij���ӷ�ˮ�м���Na2S����S2-Ũ�ȴﵽ7.9 �� 10-8mol/Lʱ��ˮ����Cd2+Ũ��Ϊ_____mol/L����֪��Ksp��CdS��=7.9 �� 10-27��Cd�����ԭ��������112������ʱ�Ƿ����ˮԴ����______����������������������
��2����п�̳�����Ҫ�ɷ�ΪZnO��������CuO��FeO��Ϊԭ�ϣ�������ȡ�Ȼ�п�ͽ���п����ȡ�Ȼ�п��Ҫ�������£�
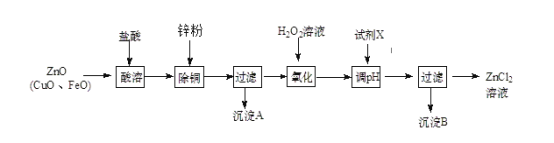
�±��г�����ؽ������������������������pH (��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
�������� | Fe3+ | Zn2+ | Fe2+ |
��ʼ������pH | 1. 1 | 5. 2 | 5. 8 |
������ȫ��pH | 3. 2 | 6. 4 | 8. 8 |
������H2O2��Һ��������________________��
������ͼ�У�����pHʱ��������Լ�X������________������ţ���
a��ZnO b��NaOH c��Zn2(OH)2CO3 d��ZnSO4
pHӦ������______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com