
ijͬѧ������һƿ��������84����Һ��������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25%NaClO 1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش��������⣺
(1)����84����Һ�������ʵ���Ũ��Ϊ________ mol��L��1��
(2)��ͬѧȡ100 mL����84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��
c(Na��)��________ mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)ijʵ������480 mL��25%NaClO������Һ����ͬѧ���ĸ���84����Һ�����䷽������NaClO�������Ƹ�����Һ��
������˵����ȷ����________��

A������ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D����Ҫ������NaClO��������Ϊ143 g
�������ƹ����У����в�������ʹ���Ƶ���Һ��Ũ��ƫ�����________��
A���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ�
B������ʱ�����ӿ̶���
C������ʱ�����ӿ̶���
D����Һʱ��������Һ�彦��
(1)4.0��(2)0.04��(3)��C����B
��������(1)����c��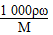 ��
��
c(NaClO)��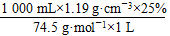 =4.0 mol��L��1
=4.0 mol��L��1
(2)ϡ��100����Ũ��Ϊԭ����1%��
(3)��ѡ��A������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ�е�����������������Ҫ�������貣�����ͽ�ͷ�ιܡ�ѡ��B�����ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�á�ѡ��C������NaClO�����տ����е�H2O��CO2�����ʣ�������ƷNaClO���ܲ��ֱ��ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�͡�ѡ��D��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO��������0.5 L��4.0 mol��L��1��74.5 g��mol��1��149 g��
����c��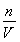 �жϣ�A��Dѡ����ʹnƫС��Ũ��ƫС��Bѡ���и��ӿ̶��ߣ�ʹVƫС��Ũ��ƫ��Cѡ�������ӿ̶��ߣ�ʹVƫ��Ũ��ƫС��
�жϣ�A��Dѡ����ʹnƫС��Ũ��ƫС��Bѡ���и��ӿ̶��ߣ�ʹVƫС��Ũ��ƫ��Cѡ�������ӿ̶��ߣ�ʹVƫ��Ũ��ƫС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��9���ǽ���Ԫ�ػ�������ϰ���������棩 ���ͣ�ѡ����
���ʼ�����ӵ�ת����ϵ�ǻ�ѧ���������ڣ�����ѡ�������ʵ�ת����һ�������²���ʵ�ֵ���(����)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��5����ѧ��Ӧ��������ϰ���������棩 ���ͣ�ѡ����
��֪����1 g��������������ȫȼ��������̬ˮ���ų�����120.9 kJ�����к���Ϊ57.3 kJ��mol��1����C(ʯīs)=C(���ʯs)����H����1.90 kJ��mol��1������˵����ȷ���� (����)��
A��������ȼ����Ϊ241.8 kJ��mol��1
B������ȼ�յ��Ȼ�ѧ����ʽ��2H2��O2=2H2O����H����483.6 kJ��mol��1
C������Ͱ�ˮ��ϵ��Ȼ�ѧ����ʽ��
H��(aq)��OH��(aq)=H2O(l)����H����57.3 kJ��mol��1
D��������֪���ʯ����ʯī�ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��3��������Ҫ�ķ�Ӧ��ϰ���������棩 ���ͣ������
(����Ԥ����)(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________�����ӷ���ʽΪ__________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ______________________________�����ӷ���ʽ��_______________________________��
���Ա�������ʵ�����õĽ������I2��ClO����SO42������ǿ������˳������Ϊ_____________________________��
(2)������Ƭ��ͭƬ�����ʵ��֤��������ʵ����д����Ӧ�Ļ�ѧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��________________________________��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��__________________________��
�����Ļ�ԭ�Ա�ͭǿ��
___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��3��������Ҫ�ķ�Ӧ��ϰ���������棩 ���ͣ�ѡ����
���й������Ӽ����˵������ȷ����(����)��
A����ij��Һ�м���AgNO3��Һ�����ɰ�ɫ����������Һ��һ������Cl��
B����ij��Һ�м���ϡ���ᣬ������ɫ���壬�����Һ��һ������CO32��
C����ij��Һ�м��������ữ��BaCl2��Һ���а�ɫ�������ɣ�����Һ��һ������SO42��
D����ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�мӼ������Ƶ���ˮ����Һ��Ϊ��ɫ������Һ��һ������Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��2����ѧ���ü�����ϰ���������棩 ���ͣ�ѡ����
��27.2 g Cu��Cu2O�Ļ�����м���ijŨ�ȵ�ϡ����0.5 L������������ȫ��Ӧ������NO��Cu(NO3)2����������Һ�м���1.0 molL��1��NaOH��Һ1.0 L����ʱ��Һ�����ԣ�������������ȫ��������������Ϊ39.2 g�������й�˵������ȷ���� (����)��
A��Cu��Cu2O�����ʵ���֮��Ϊ2��1
B����������ʵ���Ũ��Ϊ2.6 mol��L��1
C��������NO�ڱ�״���µ����Ϊ4.48 L
D��Cu��Cu2O�����ᷴӦ��ʣ��HNO3Ϊ0.2 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��1������������ʷ�����ϰ���������棩 ���ͣ������
�輰�仯����������ִ������ķ�չ��������ס���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
��������Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ����������̫���ܵ��
A���٢ڢ� B���ۢܢ� C���ڢۢ� D���٢ۢ�
(3)�����£�SiCl4ΪҺ̬���е�Ϊ57.6 �����ڿ�����ð�������Ʊ��ߴ��ȹ���м����SiCl4������Һ̬���ʣ���Ҫ�õ��ߴ���SiCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��12����ѧʵ�������ϰ���������棩 ���ͣ�ʵ����
�Ʊ�����þ��װ��ʾ��ͼ���£�

�ش��������⣺
(1)���װ�������Եķ�����_______________________________________��
a��������________��b��������________��
(2)д��NaNO2��(NH4)2SO4��Ӧ�Ʊ������Ļ�ѧ����ʽ______________________________��
(3)C��������______________________________________��
D��������_______________________________________��
�Ƿ����C��D��λ�öԵ���˵������____________________________��
(4)д��E�з�����Ӧ�Ļ�ѧ����ʽ______________________________��
(5)���û�ѧ����ȷ���Ƿ��е���þ���ɣ��������Ƿ���δ��Ӧ��þ��д��ʵ�����������__________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ ר��8�绯ѧԭ����ϰ���������棩 ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⡣
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)===2CO(g)��2H2(g)����H��260 kJ��mol��1
��֪��2CO(g)��O2(g)===2CO2(g)
��H����566 kJ��mol��1��
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_________________________________��
(2)����ͼ��ʾ��װ����Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ����ʵ�������϶�ͭ��

��a��Ӧͨ��________(����CH4������O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��__________________________________��
����ƽ�����װ��������Һ��pH________(��д�����������С����������������ͬ)��װ������Cu2�������ʵ���Ũ��________��
����ƽ�����װ������Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ���������������仯12.8 g����װ���������������ļ���________L(��״����)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com