
(����Ԥ����)(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________�����ӷ���ʽΪ__________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ______________________________�����ӷ���ʽ��_______________________________��
���Ա�������ʵ�����õĽ������I2��ClO����SO42������ǿ������˳������Ϊ_____________________________��
(2)������Ƭ��ͭƬ�����ʵ��֤��������ʵ����д����Ӧ�Ļ�ѧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��________________________________��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��__________________________��
�����Ļ�ԭ�Ա�ͭǿ��
___________________________________________________________��
(1)��NaClO��KI����������I2
ClO����2I����H2O=I2��Cl����2OH��
��I2��Na2SO3��ԭ������
SO32����2OH��=SO42������H2O
��ClO����I2��SO42��
(2)��Cu��Ũ���ᷴӦ����ϡ�����Ӧ��
Cu��2H2SO4(Ũ)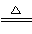 ��SO2����2H2O
��SO2����2H2O
��Cu����FeCl3��Һ��Ӧ��
2FeCl3��Cu=2FeCl2��CuCl2
��Fe����CuSO4��Һ��Ӧ�û���Cu��
Fe��CuSO4=FeSO4��Cu(�𰸲�Ψһ����������)
��������(1)���õ�����I2������������֤NaClO�������Ժ�Na2SO3�Ļ�ԭ�ԣ���д��ѧ����ʽʱע����Һ����Ϊ���ԡ�(2)�оٳ�����Ӧ��ʵ���ɡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ѵ�� Ԫ�ؼ��仯������ϰ���������棩 ���ͣ�ѡ����
����ͬ�����£�����ͬ���ʵ�����Na��Mg��Al�ֱ����ʢ��ͬŨ�ȡ�ͬ���ϡ����ļס��ҡ��������ձ��г�ַ�Ӧ����������������ϵ��������(����)
A����(Na)����(Mg)����(Al) B����(Na)����(Mg)����(Al)
C����(Na)����(Mg)����(Al) D����(Na)����(Mg)����(Al)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��6����Ӧ���ʺͻ�ѧƽ����ϰ���������棩 ���ͣ�ѡ����
��һ�ܱ������У���CO2��H2�ϳɼ״����������������������£�̽���¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ(ע��T1��T2������300 ��)������˵����ȷ����(����)��

A���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH)�� mol��L��1��min��1
mol��L��1��min��1
B���÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
C���÷�ӦΪ���ȷ�Ӧ
D������A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��5����ѧ��Ӧ��������ϰ���������棩 ���ͣ�ѡ����
���й��ڻ�ѧ��Ӧ����������ȷ����(����)��
A����Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ
B����֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l) ��H����57.3 kJ��mol��1����40.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ�Ҳ�ų�57.3 kJ������
C��CO(g)��ȼ������283.0 kJ��mol��1�����ʾCO(g)��ȼ���ȵ��Ȼ�ѧ����ʽΪ2CO(g)��O2(g)=2CO2(g)����H����283.0 kJ��mol��1
D����֪2C(s)��2O2(g)=2CO2(g)����H��a,2C(s)��O2(g)=2CO(g)����H��b����b��a
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��4�������ɡ����ʽṹ��ϰ���������棩 ���ͣ�ѡ����
��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ�����C3B2�����з�����ȷ����(����)��

A���縺�ԣ�C>A
B�����������ԣ�C>D
C���⻯���ȶ��ԣ�A>B
D��ԭ�Ӱ뾶��r(C)>r(B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��3��������Ҫ�ķ�Ӧ��ϰ���������棩 ���ͣ�ѡ����
���н�����ʵ�ķ���ʽ��ȷ����(����)��
A����Ũ������鰱��NH3��HCl=NH4Cl
B��̼������Һ�Լ��ԣ�CO32����H2O HCO3����OH��
HCO3����OH��
C����������������ʴʱ������������������Fe��3e��=Fe3��
D������ʢ��ʯ��ˮ���Լ�ƿ�ڱڳ��ְ�ɫ���壺Ca(OH)2��CO2=CaCO3����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��2����ѧ���ü�����ϰ���������棩 ���ͣ������
ijͬѧ������һƿ��������84����Һ��������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25%NaClO 1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش��������⣺
(1)����84����Һ�������ʵ���Ũ��Ϊ________ mol��L��1��
(2)��ͬѧȡ100 mL����84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��
c(Na��)��________ mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)ijʵ������480 mL��25%NaClO������Һ����ͬѧ���ĸ���84����Һ�����䷽������NaClO�������Ƹ�����Һ��
������˵����ȷ����________��

A������ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D����Ҫ������NaClO��������Ϊ143 g
�������ƹ����У����в�������ʹ���Ƶ���Һ��Ũ��ƫ�����________��
A���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ�
B������ʱ�����ӿ̶���
C������ʱ�����ӿ̶���
D����Һʱ��������Һ�彦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ�Ĵ�����ѵ�� ��1������������ʷ�����ϰ���������棩 ���ͣ�ѡ����
�����й����ʷ���һ����ȷ����(����)��
��ǿ����ʣ��Ȼ��⡢����������Ħ���Ρ���������ʣ����ᡢ�������李����ǵ���ʣ�Һ����������������ͬϵ�CH2O2��C2H4O2��C3H6O2
A���٢ڢ� B���٢� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ ר��9��������Ԫ�ؼ�����Ҫ��������ϰ���������棩 ���ͣ������
����ת����ϵ�У�X��Y����������;�㷺�����ֽ������ʣ�A��B�������A�ʺ���ɫ��C��D��E����ѧ���������ֻ��������ת����ϵ�ش����⣺

(1)��д����Ӧ���Ļ�ѧ����ʽ_____________________________________��
(2)����D��Һ��Y���ӵķ�����__________________________________��
(3)���Լ�a��NaOH��Һ��д������X��NaOH��Һ��Ӧ�����ӷ���ʽ______________________________��
(4)���Լ�b��H2SO4����ҵ����E��H2SO4��NaNO2Ϊԭ����ȡ��Ч��ˮ��Y(OH)SO4����֪��ԭ����ΪNO����÷�Ӧ�Ļ�ѧ����ʽ��________________________________________��
(5)��ҵ�ϵ�����ڵ�B��ȡXʱ�������������������ڱ�״���µ����Ϊ33.6 m3�����������������Ϊ________kg��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com