

 2NaOH+H2��+Cl2������ 12
2NaOH+H2��+Cl2������ 12  2NaOH+H2��+Cl2������ 12
2NaOH+H2��+Cl2������ 12  2NaOH+H2��+Cl2������B������Cl2��11.2mL/22400ml��mol-1=0.0005mol,�ɷ���ʽ������0.001molNaOH ,c(NaOH)=0.001mol/0.1L=0.01mol / L,pH="14+lg0.01" ="12"
2NaOH+H2��+Cl2������B������Cl2��11.2mL/22400ml��mol-1=0.0005mol,�ɷ���ʽ������0.001molNaOH ,c(NaOH)=0.001mol/0.1L=0.01mol / L,pH="14+lg0.01" ="12" 

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
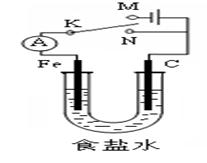
| A�������K��N���ӣ������������ḯʴ |
| B�������K��N���ӣ�������Ӧʽ��4OH- -4e-=2H2O+O2�� |
| C�������K��M���ӣ���ʯī������ͭ����������������ͭ���� |
| D�������K��M���ӣ�������������22.4 L(��״��)����ʱ��������1 molNaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ͭƬ���ӵ�Դ����������һ�缫�ò�Ƭ |
| B����̼�����ӵ�Դ����������һ�缫��ͭƬ |
| C��������������Һ������������ |
| D���ô����ǵ�ľ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
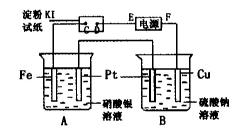
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 _______
_______  ______��
______���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������,�����������������������ͬ(ͬ��ͬѹʱ) |
| B���������Һ��c(NaOH)=1mol/L |
| C����������,ת�Ƶ��ӵ����ʵ���ԼΪ8mol |
| D��ԭ��Һ�к���117g NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
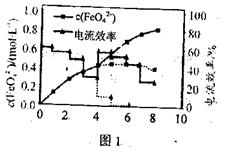
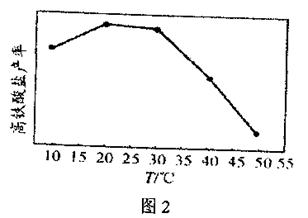
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com