
| |||||||||||||||||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
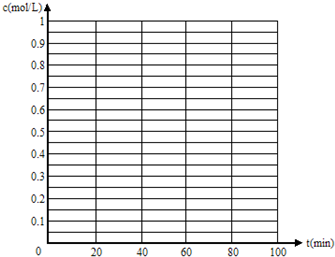
| 0min | 10min | 20min | 30min | 40min | 50min | |
| 830�� | 1mol | 0.8mol | 0.65mol | 0.55mol | 0.5mol | 0.5mol |
| 1100�� | 1mol | 0.75mol | 0.6mol | 0.6mol | 0.6mol | 0.6mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ����и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
�����Ϊ1L�����������ܱ������У��ֱ����1molCO��1molH2O(g)�Ļ�����壬�������»�ѧ��Ӧ��CO(g)��H2O(g)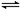 CO2(g)��H2(g)����Ӧ��CO�����ʵ����ڲ�ͬ�¶�ʱ���ʵ�����ʱ��ı仯���±���ʾ���ش��������⣺
CO2(g)��H2(g)����Ӧ��CO�����ʵ����ڲ�ͬ�¶�ʱ���ʵ�����ʱ��ı仯���±���ʾ���ش��������⣺
| | 0min | 10min | 20min | 30min | 40min | 50min |
| 830�� | 1mol | 0.8mol | 0.65mol | 0.55mol | 0.5mol | 0.5mol |
| 1100�� | 1mol | 0.75mol | 0.6mol | 0.6mol | 0.6mol | 0.6mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ģ���� ���ͣ������
 Na2S(s)+4H2O(g)�����ﵽƽ�⡣��������и��⣺
Na2S(s)+4H2O(g)�����ﵽƽ�⡣��������и��⣺ 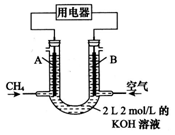
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.��֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����
![]()
![]()
������ ��![]() ��?H��______________��
��?H��______________��
��.1100��ʱ�������Ϊ2L�ĺ��������з������·�Ӧ��
Na2SO4��s��+4H2(g) Na2S(s)+4H2O(g).
(1)�������жϸ÷�Ӧ�ﵽƽ��״̬������������������ ��
A��������ѹǿ���䡡�������������������� �� B����������ܶȲ���
C��1molH-H�����ѵ�ͬʱ�γ�2molH-O���� �� D��H2�������������
��2����2minʱ��Ӧ�ﵽƽ�⣬��ʱ��������������8g������H2��ʾ�÷�Ӧ�Ļ�ѧ��Ӧ����Ϊ�������������������������� ��
��3��ij�¶��¸÷�Ӧ�ﵽƽ��״̬����û�������ƽ����Է�������Ϊ14������¶��µ�ƽ�ⳣ��KΪ���������������� ��
��4���������¶ȣ�Kֵ��С����Ӧ�ġ�H���� �������������0��
��5������Ӧ��ƽ�������ϵ�м���������H2����Ӧ�ٴδ�ƽ���H2?O��g������������������������������������������������������� �����������С�����䡱����
��ij500mL�����Һ�к�0.1molNaCl��0.1molCuSO4���ں㶨����������½��е�⣬2min��������ʼ�������壬5minʱֹͣ��⣬��ʱ�������������������������ͬ��
��д���˹����������ĵ缫��Ӧʽ________________��
_____________________________��
�ڽ���Һϡ��Ϊ1000ml��������Һ��pHΪ�������� ��
������0.1 ��CrCl3 ��Һ�м�����������Һ����Cr(OH)3���������������£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH�D)=10-32��Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ������������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com