
 2015��8��12�Žӽ���ҹʱ�֣�����������һ����װ����ͷ������ը��������ը���Ǽ�װ���ڵ���ȼ�ױ���Ʒ����ը������죬��������Ģ���ƣ��������յ���Ϣ������װ������Σ�ջ�ѧƷ�����мء��ơ������ơ�����ء��ռ��軯�ơ�������ϩ���ȵ���ȣ��˵�����Σ�ջ�ѧƷ�����л����������������ᡢ����李��軯�ơ�4��6-��������-���ٶ������ӵȣ�
2015��8��12�Žӽ���ҹʱ�֣�����������һ����װ����ͷ������ը��������ը���Ǽ�װ���ڵ���ȼ�ױ���Ʒ����ը������죬��������Ģ���ƣ��������յ���Ϣ������װ������Σ�ջ�ѧƷ�����мء��ơ������ơ�����ء��ռ��軯�ơ�������ϩ���ȵ���ȣ��˵�����Σ�ջ�ѧƷ�����л����������������ᡢ����李��軯�ơ�4��6-��������-���ٶ������ӵȣ����� ��1��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
��2����ˮ�����γ�������������ʵ��ܽ�ȣ��ռ��������Ӻ����������ɣ��������Ӿ��壻S2-���Ӻ��������Ϊ18�������������ԭ������д���̬�����Ų�ʽ��
��3��NO3-�����е�ԭ�ӵŵ��Ӷ���=$\frac{5+1-2��3}{2}$=0���۲���Ӷ���=3+0=3��
��4��ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ���ӣ�CN-��N2��Ϊ�ȵ����壬CN-�к���C��N�����������к���1���Ҽ���2���м�����������ȡ�����ķ�Ӧ��֪���軯�ơ��������̺�Ũ�����ڼ����������Ƶã�CN��2�������������̡���������ˮ��
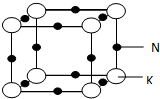
��5�����ݾ�̯�����㾧����Na��Kԭ����Ŀ��ȷ���Ͻ�Ļ�ѧʽ�����ݾ���ͼ��֪��ÿ��K ԭ����Χ��6����ԭ�ӣ����ݾ����Ľṹ��֪�������ı߳�Ϊ��ԭ�Ӻͼ�ԭ�ӵ�ֱ��֮�ͣ�����Ŀռ�������Ϊ$\frac{������Na��Kԭ�������}{�������}$��100%��
��� �⣺��1��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ�����Ԫ�صģ���NH4NO3��NaCN�������ʵ�Ԫ���е�һ������������N��
�ʴ�Ϊ��N��ͬ����Ԫ�ص�һ������������ҳ��������ƣ�ͬ�������϶���Ԫ�ص�һ��������С����Nԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ�����Ԫ�صģ�
��2��������ˮ�γ�����������������ܣ��ʶ�������ͼ������ܽ�Ƚϴ���Ǽ��ᣬ�ռ��������Ӻ����������ɣ������ռ������Ӿ��壬S2-���Ӻ�����18�����ӣ����̬�����Ų�ʽΪ1s2s22p63s23p6��
�ʴ�Ϊ�����������ˮ�γ���������Ӿ��壻1s2s22p63s23p6��
��3��NO3-�����е�ԭ�ӵŵ��Ӷ���=$\frac{5+1-2��3}{2}$=0���۲���Ӷ���=3+0=3������NO3-���幹��Ϊƽ�������Σ�����ԭ�ӵ�ԭ�ӵ��ӻ��������sp2��
�ʴ�Ϊ��ƽ�������Σ�sp2��
��4��ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ���ӣ�CN-��N2��Ϊ�ȵ����壬���߽ṹ���ƣ�CN-�к���C��N�����������к���1���Ҽ���2���м���������1mol������NaCN��CN-�����Ħм���Ϊ2NA��CN-�к�������ԭ�ӡ�10���۵��ӣ���CN-��Ϊ�ȵ�����ķ�����CO��N2��
������ȡ�����ķ�Ӧ��֪���軯�ơ��������̺�Ũ�����ڼ����������Ƶã�CN��2����Ӧ��ѧ����ʽΪ��2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$��CN��2+Na2SO4+MnSO4+2H2O��
�ʴ�Ϊ��2NA��CO��N2��2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$��CN��2+Na2SO4+MnSO4+2H2O��
��5�������У���ԭ����Ϊ12��$\frac{1}{4}$=3����ԭ����Ϊ8��$\frac{1}{8}$=1�����ԺϽ�Ļ�ѧʽΪKNa3��
���ݾ���ͼ��֪��ÿ��K ԭ����Χ��6����ԭ�ӣ����Ծ�����K ԭ�ӵ���λ��Ϊ6��
��������ԭ�Ӻͼ�ԭ�����֮��Ϊ$\frac{4}{3}$��[��186pm��3��3+��227pm��3]�������ı߳�Ϊ��ԭ�Ӻͼ�ԭ�ӵ�ֱ��֮��Ϊ2����186pm+227pm�������Ծ��������Ϊ��2��186pm+2��227pm��3������Ŀռ�������Ϊ
{$\frac{4}{3}$��[��186pm��3��3+��227pm��3]�£�2��186pm+2��227pm��3}��100%=$\frac{\frac{4}{3}�У�18{6}^{3}��3+22{7}^{3}��}{��186��2+227��2��^{3}}$��100%��
�ʴ�Ϊ��KNa3��6��$\frac{\frac{4}{3}�У�18{6}^{3}��3+22{7}^{3}��}{��186��2+227��2��^{3}}$��100%��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰�����ܡ���������Ų����ӻ���ʽ��ռ乹�͡��ȵ����塢��������ȣ��Ѷ��еȣ�������ѧ���ķ��������ͼ��������Ŀ��飬ע�����֪ʶ���������գ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���ʳ��ˮ���ռ� | B�� | �ϳɰ��еĴ��ϳ� | ||
| C�� | ���������еĴ����� | D�� | ����еİ���ˮ̼�ữ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
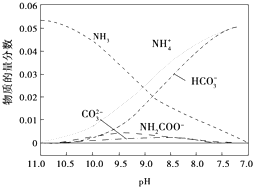
| A�� | ��pH=9.0ʱ��c��NH4+����c��HCO3-����c��NH2COO-����c��CO32-�� | |
| B�� | ��ͬpH����Һ�д��ڹ�ϵ��c��NH4+��+c��H+���T2c��CO32-��+c��HCO3-��+c��NH2COO-��+c��OH-�� | |
| C�� | ����ҺpH���Ͻ��͵Ĺ����У��к�NH2COO-���м�������� | |
| D�� | ����CO2��ͨ�룬$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
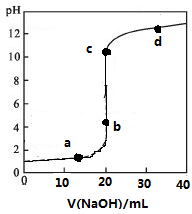 �����£���20mL0.1mol/Lij��HR��Һ�еμ�0.1mol/L����������Һ����Һ��PH������������Һ���V�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
�����£���20mL0.1mol/Lij��HR��Һ�еμ�0.1mol/L����������Һ����Һ��PH������������Һ���V�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ��ѡ��̪�������ָʾ�� | |
| B�� | �ζ�ǰHR��Һ�д��ڴ���HR���� | |
| C�� | V=20 mLʱ����Һ��ˮ����ģ�c��H+����c��OH-��=1��10-14mol2/L2 | |
| D�� | c��ʱ��Һ������Ũ�ȴ�С��ϵ��c��Na+����c��R-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
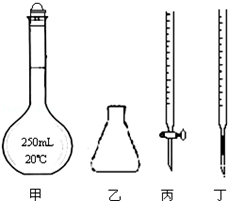 ���������һ����Ҫ�Ļ�ѧ�Լ�������Һ�����ȶ��������������»�ֽ����ɶ������̺������������Ի���������Һ�зֽ��ٶȺ���������ֽ��ٶȼӿ죮
���������һ����Ҫ�Ļ�ѧ�Լ�������Һ�����ȶ��������������»�ֽ����ɶ������̺������������Ի���������Һ�зֽ��ٶȺ���������ֽ��ٶȼӿ죮������������Һ�Ĵ���ÿ����Һ�������ͬ�� | ���������Һ��ɫ��ȥ��ʱ�� |
| �ȵ����1�� | 1min |
| ��ɫ���ٵ����2�� | 15s |
| ��ɫ���ٵ����3�� | 3s |
| ��ɫ���ٵ����4�� | 1s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
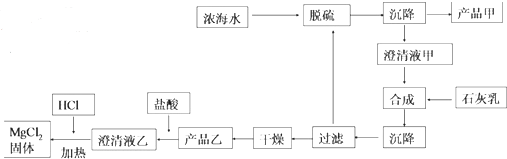
| ���� | Na+ | Mg2+ | Cl- | SO${\;}_{4}^{2-}$ |
| Ũ��/��g•L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��Na+��=c��HA-��+2c��A2-��+c��OH-�� | |
| B�� | c��H2A��+c��HA-��+c��A2-��=0.1 mol•L-1 | |
| C�� | ��������Һϡ����0.01mol/L��c��H+��•c��OH-�� ���� | |
| D�� | c ��A2-��+c ��OH-��=c ��H+��+c ��H2A�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
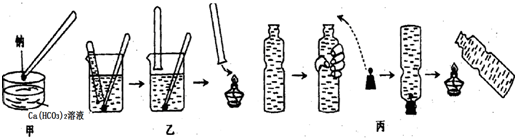
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

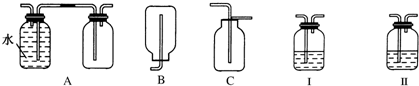
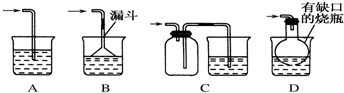
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com