
����Ŀ�����ǹ�ũҵ��������Ҫ�Ļ������ʣ��о��ϳɰ�������Ӧ�þ�����Ҫ���塣
��1����֪��N2(g)+3H2(g)=2NH3(g)��H=-92kJ/mol��N2(g)+3H2(g)![]() 2NH3(g)�Ļ��Ϊ508kJ/mol����2NH3(g)
2NH3(g)�Ļ��Ϊ508kJ/mol����2NH3(g)![]() N2(g)+3H2(g)�Ļ��Ϊ___________kJ/mol
N2(g)+3H2(g)�Ļ��Ϊ___________kJ/mol
��2���ҹ�������Ա���Ƴ�Ti-H-Fe˫����������Ti-H����Fe���²�ɳ���100�棩��Ti-H-Fe˫�������ϳɰ��ķ�Ӧ��������ͼһ�����������ڴ�������������á�*����ע��
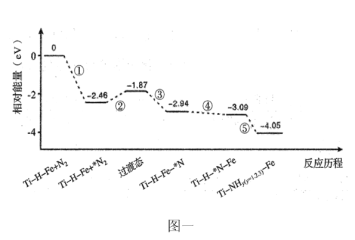
����˵������ȷ����___________��
A����Ϊ![]() �Ķ��ѹ���
�Ķ��ѹ���
B���٢ڢ��ڸ������������ܢ��ڵ���������
C����ΪNԭ����Fe������Ti-H����Ĵ��ݹ���
D��ʹ��Ti-H-Fe˫��������ʱ�ϳɰ���Ӧת��Ϊ���ȷ�Ӧ
��3������CO2Ϊԭ�Ϻϳ����صķ�ӦΪ2NH3(g)��CO2(g)![]() CO(NH2)2(l)��H2O(g)����ҵ����ʱ����Ҫԭ��������ˮ������ͼ�������ߢ��ʾ�ڲ�ͬˮ̼��[
CO(NH2)2(l)��H2O(g)����ҵ����ʱ����Ҫԭ��������ˮ������ͼ�������ߢ��ʾ�ڲ�ͬˮ̼��[![]() ]ʱ��CO2��ƽ��ת�����백̼��[
]ʱ��CO2��ƽ��ת�����백̼��[![]() ]֮��Ĺ�ϵ��
]֮��Ĺ�ϵ��
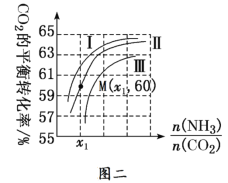
�����ߢ��Ӧ��ˮ̼��������________���ж�������________��
�ڲ��M�㰱����ƽ��ת����Ϊ40%����x1��______��
��4����������������������а���������������壨����800�棩������ȴ��100�����£�һ�����ȥ������H2O��ʹNO(g)��O2(g)������Ӧ����һ�����¶ȵ�����������NO2(g)��
2NO(g)��O2(g)![]() 2NO2(g)�ķ�Ӧ���̷�������
2NO2(g)�ķ�Ӧ���̷�������
��.2NO(g)![]() N2O2(g)(��Ӧ�죬˲��ﵽƽ��)��H1��0
N2O2(g)(��Ӧ�죬˲��ﵽƽ��)��H1��0
v1����k1��c2(NO) v1����k1��c(N2O2)
��.N2O2(g)��O2(g)![]() 2NO2(g)(��Ӧ��)��H2��0
2NO2(g)(��Ӧ��)��H2��0
v2����k2��c(N2O2)c(O2) v2����k2��c2(NO2)
����k1��k2�����ʳ��������¶�����������
��:��һ���¶��£���Ӧ2NO(g)��O2(g)![]() 2NO2(g)�ﵽƽ��״̬����д����k1����k1����k2����k2����ʾ��ƽ�ⳣ������ʽK��_____���������ʷ��̷����������¶ȸ��ܷ�Ӧ���ʼ�С��ԭ����__________��
2NO2(g)�ﵽƽ��״̬����д����k1����k1����k2����k2����ʾ��ƽ�ⳣ������ʽK��_____���������ʷ��̷����������¶ȸ��ܷ�Ӧ���ʼ�С��ԭ����__________��
����ʵ�����ݵõ�v2����c(O2)�Ĺ�ϵ������ͼ��ʾ����x�����ߵ�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬����ܱ�Ϊ��Ӧ�ĵ�Ϊ__(����ĸ)��

���𰸡�600 BC �� ����̼����ͬʱ��ˮ̼��Խ��ԭ�����к�ˮ����Խ�࣬CO2��ƽ��ת����ԽС 3 ![]() �¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�����ʱ�С���ܷ�Ӧ�������ɽ�����һ�������������ܷ�Ӧ���ʼ�С a
�¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�����ʱ�С���ܷ�Ӧ�������ɽ�����һ�������������ܷ�Ӧ���ʼ�С a
��������
(1)��N2(g)+3H2(g)![]() 2NH3(g)�Ļ��ΪE1��2NH3(g)
2NH3(g)�Ļ��ΪE1��2NH3(g)![]() N2(g)+3H2(g)�Ļ��ΪE2��2NH3(g)
N2(g)+3H2(g)�Ļ��ΪE2��2NH3(g)![]() N2(g)+3H2(g)��N2(g)+3H2(g)
N2(g)+3H2(g)��N2(g)+3H2(g)![]() 2NH3(g)���淴Ӧ����E1-E2=��H�����ԣ�508kJ/mol -E2=-92kJ/mol����ã�E2=600kJ/mol���ʴ�Ϊ��600��
2NH3(g)���淴Ӧ����E1-E2=��H�����ԣ�508kJ/mol -E2=-92kJ/mol����ã�E2=600kJ/mol���ʴ�Ϊ��600��
(2)A����Ϊ��������N2�Ĺ��̣�A����
B����ͼ�Ͽ�֪���ڹ���Ϊ��Fe���γɹ���̬�Ĺ��̣�Ϊ���ȷ�Ӧ���ڸ�������Ӧ�������γɹ���̬����ôFe��Ϊ��������Ti-H��Ϊ���������٢ڢ۶�����Fe����Ӧ�ģ����٢ڢ۶����ڸ�������Ӧ�ģ��ܢݶ�����Ti-H����Ӧ�ģ����ܢݶ����ڵ�������Ӧ�ģ�B��ȷ��
C����ͼ��֪����ΪNԭ����Fe������Ti-H����Ĵ��ݹ��̣�C��ȷ��
D��һ����Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ���Ƿ�ʹ�ô�����ʹ��ʲô�����أ�ֻ�뷴Ӧ���ʼ̬����̬�йأ��ɺϳɰ�����H��0��֪���ϳɰ�Ϊ���ȷ�Ӧ��D����
����������BC��ȷ��AD���ʴ�Ϊ��BC��
(3)�ٺ����꣬����̼��[![]() ]һ��ʱ��ˮ̼��[
]һ��ʱ��ˮ̼��[![]() ]������n(H2O)����c(H2O)����ƽ�������ƶ���CO2��ת����Ӧ��С������̼����ͬʱ��ˮ̼��Խ��ԭ�����к�ˮ����Խ�࣬CO2��ƽ��ת����ԽС�����Ϊˮ̼���������ߣ��ʴ�Ϊ������̼����ͬʱ��ˮ̼��Խ��ԭ�����к�ˮ����Խ�࣬CO2��ƽ��ת����ԽС��
]������n(H2O)����c(H2O)����ƽ�������ƶ���CO2��ת����Ӧ��С������̼����ͬʱ��ˮ̼��Խ��ԭ�����к�ˮ����Խ�࣬CO2��ƽ��ת����ԽС�����Ϊˮ̼���������ߣ��ʴ�Ϊ������̼����ͬʱ��ˮ̼��Խ��ԭ�����к�ˮ����Խ�࣬CO2��ƽ��ת����ԽС��
����CO2����ʼ���ʵ���Ϊ1����NH3����ʼ���ʵ���Ϊx1��M�㣺������ƽ��ת����Ϊ40%��CO2��ת����Ϊ60%�����У�40%x1=60%��1��2�����x1=3���ʴ�Ϊ��3��
(4)��ƽ��ʱ��v1����v1����v2����v2������k1��c2(NO)=k1��c(N2O2)��k2��=c(N2O2)��c(O2)��k2��c2(NO2)����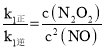 ��
��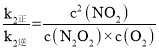 ��2NO(g)��O2(g)
��2NO(g)��O2(g)![]() 2NO2(g)��K=
2NO2(g)��K=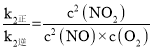 =
=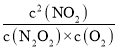 ��
�� =
=![]() ��
��![]() =
=![]() ���¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�������ʱ�С���ܷ�Ӧ�������ɽ�����һ�������������ܷ�Ӧ���ʼ�С���ʴ�Ϊ��
���¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�������ʱ�С���ܷ�Ӧ�������ɽ�����һ�������������ܷ�Ӧ���ʼ�С���ʴ�Ϊ��![]() ���¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�����ʱ�С���ܷ�Ӧ�������ɽ�����һ�������������ܷ�Ӧ���ʼ�С��
���¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�����ʱ�С���ܷ�Ӧ�������ɽ�����һ�������������ܷ�Ӧ���ʼ�С��
���¶�����ƽ��N2O2(g)��O2(g)![]() 2NO2(g)�����ƶ���c(O2)���������x��ĺ�������¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�����ʱ�С����v2����С���������x���������С������������a����ܣ��ʴ�Ϊ��a��
2NO2(g)�����ƶ���c(O2)���������x��ĺ�������¶����ߣ���ӦI�����ƶ���Ѹ�ٴﵽƽ�⣬����N2O2Ũ�ȼ�С����Ȼ�¶�����k2����k2���������Է�Ӧ���Ӱ������N2O2Ũ�ȼ�С��Ӱ�죬���·�Ӧ�����ʱ�С����v2����С���������x���������С������������a����ܣ��ʴ�Ϊ��a��


 ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(ClO2������ɫ������ˮ������)��һ�ְ�ȫ�ȶ�����Ч�Ͷ�������������ҵ��ͨ�����Ե缫����Ȼ�狀�����ķ����Ʊ�����ԭ����ͼ��ʾ:

����˵������ȷ����
A. b�缫�ӵ�Դ�ĸ�������b����������Y��Һ��ϡ����
B. �������ȷ��������ų���X��Һ��������ҪΪNaCl��NaOH
C. �������ж������ȷ������в���2.24L(��״��)NH3����b������0.6gH2
D. ����a���ĵ缫��ӦʽΪNH4+-6e-+4OH-+3Cl-=NCl3+4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ����й����߽�����������(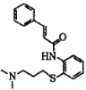 �����ܶ� 2019- nCoV����Ч������ĺϳ�·����ͼ��
�����ܶ� 2019- nCoV����Ч������ĺϳ�·����ͼ��

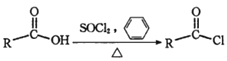
��֪��
��
![]()

��R-NO2![]() R-NH2
R-NH2
�����������
(1)F�Ľṹ��ʽΪ__________��G��H �ķ�Ӧ������____________��
(2)E�е���������������Ϊ______________��
(3)B��C��Ӧ�Լ�������Ϊ_______________ ��
(4)�л��� D ������������ͭ����Һ�����ȣ��ķ�Ӧ����ʽΪ_________��
(5)JΪ E��ͬ���칹�壬д����������������J �Ľṹ��ʽ_______��д���֣���
���ܷ���������Ӧ
��J �Ƿ����廯����ұ����ϵ�һ�ȴ���ֻ������
�ۺ˴Ź��������� 5 ��壬�ҷ����֮��Ϊ 2 ��2 ��2 ��1 ��1
(6)���������ϳ�·����֪��Ϣ��д���ɼױ��ͱ�����(CH3CH2COCl) Ϊԭ�Ϻϳ� ��·�ߣ�___________��
��·�ߣ�___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25�棬��0.1000mol/L��NaOH��Һ�ζ�40.00mL0.1mol/L��ij��Ԫ����H2R��Һ�����õζ�������ͼ��ʾ����������Һ�������������Һ���֮�ͣ�������˵����ȷ����
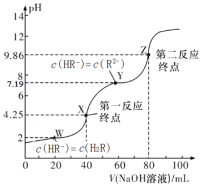
A.��ֻ�ζ�����һ��Ӧ�յ㣬���÷�̪��ָʾ��
B.ͼ��Y���Ӧ����Һ�У�c(Na+)+c(H+)=2c(HR-)+c(OH-)
C.ͼ��Z���Ӧ����Һ��c(Na+)ԼΪ0.067mol/L��R2-��ˮ���ʴ���1%
D.��pK��-lgK��2HR��![]() R2-+H2R��ƽ��ʱpK��5.19
R2-+H2R��ƽ��ʱpK��5.19
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��100 mL 0.3 mol��L��1 Na2SO4��Һ��50 mL 0.2 mol��L��1 Al2(SO4)3��Һ��Ϻ���Һ��SO42-�����ʵ���Ũ��Ϊ(����)
A. 0.20 mol��L��1B. 0.25 mol��L��1
C. 0.40 mol��L��1D. 0.50 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��B��N��F��Ga��As����һ��̫���ܵ�ء��뵼������к��е���ҪԪ�ء��ش��������⣺
��1����̬Gaԭ�ӵĺ�������Ų�ʽ��__________,��̬Gaԭ�Ӻ������ռ������ܼ��ĵ���������ͼΪ________��
��2���ڵ��������У����̬Asԭ�Ӻ���δ�ɶԵ�����Ŀ��ͬ��Ԫ��Ϊ__________��
��3��NF3�����幹��Ϊ_______��N2F2�����и�ԭ�Ӷ�����8���ӽṹ�������������������ĸ�����Ϊ______����ԭ�ӵ��ӻ��������Ϊ__________��
��4��B��Al��Ga���ʵ��۵�����Ϊ2300��C��660��C��29.8��C�������۵���������ԭ��______��
��5����B��N��F��ɵ�ij���ӻ������У�B��N��F��ԭ�Ӹ�����Ϊ1��1��8�������������ӻ�Ϊ�ȵ����壬�û������е������ӡ������ӷ��ŷֱ�Ϊ__________��
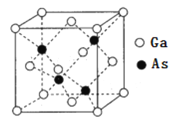
��6��GaAs����ṹ��ͼ��ʾ��
��ͼ��Asԭ�ӵ��������Ϊ![]() ��_______��
��_______��
����֪�����������������Ga��Asԭ�ӵĺ˼��Ϊacm��NAΪ�����ӵ�������ֵ��������ܶ�Ϊ___________g/cm3(��д����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ��һ����Ҫ�Ļ���ԭ�ϣ����ɶ���������Ʊ����ش��������⣺
��1�������飨C4H10��������1-��ϩ��C4H8�����Ȼ�ѧ����ʽ���£�
��C4H10(g)= C4H8(g)+H2(g) ��H1
��֪����C4H10(g)+ ![]() O2(g)= C4H8(g)+H2O(g) ��H2=-119 kJ��mol-1
O2(g)= C4H8(g)+H2O(g) ��H2=-119 kJ��mol-1
��H2(g)+ ![]() O2(g)= H2O(g) ��H3=-242 kJ��mol-1
O2(g)= H2O(g) ��H3=-242 kJ��mol-1
��Ӧ�ٵĦ�H1Ϊ________ kJ��mol-1��ͼ��a���Ƿ�Ӧ��ƽ��ת�����뷴Ӧ�¶ȼ�ѹǿ�Ĺ�ϵͼ��x_____________0.1������ڡ���С�ڡ�������ʹ��ϩ��ƽ�������ߣ�Ӧ��ȡ�Ĵ�ʩ��__________�����ţ���
A�������¶� B�������¶� C������ѹǿ D������ѹǿ
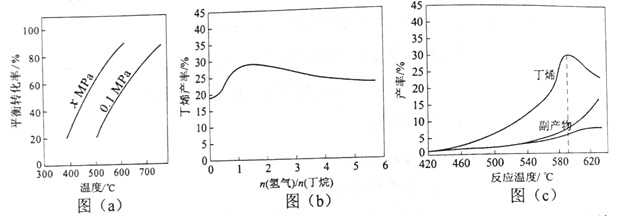
��2������������Ļ��������һ������ͨ������д����ķ�Ӧ���������������ǻ���������������к��ж�ϩ�����顢�����ȡ�ͼ��b��Ϊ��ϩ�������������n��������/n�����飩�Ĺ�ϵ��ͼ�����߳��������ߺ͵ı仯���ƣ��併�͵�ԭ����___________��
��3��ͼ��c��Ϊ��Ӧ���ʺͷ�Ӧ�¶ȵĹ�ϵ���ߣ���������Ҫ�Ǹ����ѽ����ɵĶ�̼����������ϩ������590��֮ǰ���¶����߶������ԭ�������___________��____________��590��֮��ϩ���ʿ��ٽ��͵���Ҫԭ�������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ƣ�Na2FeO4)��ˮ����������ʹ�õ�һ�����;�ˮ�������������Աȸ�����ظ�ǿ��ʵ������ȡ�������ƵĻ�ѧ����ʽ���� ��
(1)����˫���ŷ�������˷�Ӧ�ĵ���ת�������__________
2Fe( NO3 )3+16NaOH+3Cl2=2Na2FeO4+6NaNO3 +6NaCl+8H2O
(2)��Ҫ��ȡ8.3g����������Ҫ����Cl2�����Ϊ���٣�______����������������Һ���Ϊ200mL��������������Һ�����ʵ���Ũ���Ƕ��٣���д��������̣�_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��28g CO������1mol��������ȼ�պ����õ�����ͨ������Na2O2�����г�ַ�Ӧ������˵������ȷ���ǣ� ��
A. CO��������Ӧ��ֻ������0.5mol����
B. ��ַ�Ӧ��Na2O2��������������28 g
C. ͨ������������Ӧ�������������ʵ���Ϊ0.5mol
D. ��2gH2��� 28g��CO����������Ӧ����Na2O2��������������2g
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com