
 =
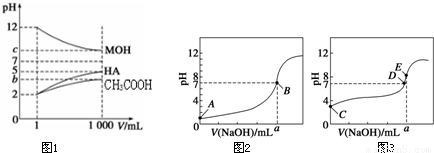
= ��ƽ������n��H+������n��CH3COOH����С���ʱ�ֵ����B��ȷ��
��ƽ������n��H+������n��CH3COOH����С���ʱ�ֵ����B��ȷ��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 CH3COOH+OH-
CH3COOH+OH- CH3COOH+OH-
CH3COOH+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |

| KMnO4 |
| H+ |










 ��
��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
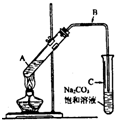 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O| ����� | ���� | ��Ӧ���� | C�б���̼������Һ������߶� |
| �� | 2mL98%Ũ���� | 20��ʱ��Һ������ɫ���淴Ӧ���У���Һ��ɫ������ɺ�ɫ�����������ݣ� | 2.10cm |
| �� | 2mL14mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.14cm |
| �� | 2mL10mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.16cm |
| �� | 2mL7mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ�����ݣ� | 2.00cm |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���¿α�2011�����ѧ�ڸ���һ�ָ�ϰ��Ԫ����1�����˽̣� ���ͣ�ѡ����
�ڴ��������������ͨ��������Ӧ�����飬��Ӧԭ�����£�
��CH3COOH+2H2��CH3CH2OH+H2O
��CH3CH2OH+H2��CH3CH3+H2O
��CH3COOH+3H2��CH3CH3+2H2O
�ڷ�Ӧ������������Ҵ�����������������Ϊ�о��ֱ��˼�Mo���⣩�Ͳ���Mo������Ni������������Ч�ܣ�ij�����ص��������£�
|
|
����ת���� |
δ��Ӧ�ĺ��������ﺬ�� |
||||
|
���� |
�Ҵ� |
|||||
|
�¶�/�� |
Mo16Ni6 |
Ni6 |
Mo16Ni6 |
Ni6 |
Mo16Ni6 |
Ni6 |
|
240 |
87.2 |
21.8 |
0.53 |
3.24 |
1.03 |
0.14 |
|
260 |
89.1 |
27.2 |
0.45 |
3.02 |
0.99 |
0.19 |
����˵������ȷ���� �� ��
A�������٢ڢ�����Ӧ�����ڼӳɷ�Ӧ
B�������٢ڢ�����Ӧ�����ڻ�ԭ��Ӧ
C���������������Ӧ260���240���½��и�����
D��Mo16Ni6�����ܱ�Ni6��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com