
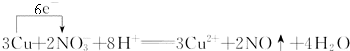 ��
��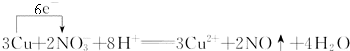 ��
��| a-b |
| a |
| a-b |
| a |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ2007��߿���ѧģ������ ���ͣ�022
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����������ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ������
�ⶨͭ�Ͻ���ͭ�ĺ�����������ϡ�������ܽ���Ʒ��Ҳ������˫��ˮ��ϡ�����ܽ���Ʒ���䷴Ӧ�Ļ�ѧ����ʽΪ��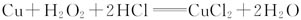 ��������Ʒ�������ɷֲ���ϡ���ᡢ˫��ˮ�����ᷴӦ��
��������Ʒ�������ɷֲ���ϡ���ᡢ˫��ˮ�����ᷴӦ��
��д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ���������ת�Ƶķ������Ŀ____________________��
����ͭ��˫��ˮ������ķ�Ӧ����������______________________________������2 mol��H2O2�μӷ�Ӧ�������ת�Ƶ����ʵ�����_______________��
����˫��ˮ��ϡ�����ܽ���Ʒ����ϡ�����ܽ���Ʒ��Ƚϣ����ָ��ã�Ϊʲô��____________________��
������ȡ��Ʒ������Ϊa g����������˫��ˮ�ܽ��ʣ���������ϴ�Ӹ���Ƶ�������b g��
����Ʒ�к�ͭ����������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 1.2��ѧʵ�����ɫ����ϰ���������棩 ���ͣ������
�ⶨͭ�Ͻ���ͭ�ĺ�����������ϡ�������ܽ���Ʒ��Ҳ������˫��ˮ��ϡ�����ܽ���Ʒ���䷴Ӧ�Ļ�ѧ����ʽΪ��Cu��H2O2��2HCl===CuCl2��2H2O��������Ʒ�������ɷֲ���ϡ���ᡢ˫��ˮ�����ᷴӦ��
(1)д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ���������ת�Ƶķ������Ŀ________________________________________________________________________��
(2)��ͭ��˫��ˮ������ķ�Ӧ����������________������2 mol��H2O2�μӷ�Ӧ�������ת�Ƶ����ʵ�����______________��
(3)��˫��ˮ��ϡ�����ܽ���Ʒ����ϡ�����ܽ���Ʒ��Ƚϣ����ָ��ã�Ϊʲô��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶�5���¿���ѧ�Ծ��������棩 ���ͣ������
�ⶨͭ�Ͻ���ͭ�ĺ�����������ϡ�������ܽ���Ʒ��Ҳ������˫��ˮ��ϡ�����ܽ���Ʒ���䷴Ӧ�Ļ�ѧ����ʽΪ�� ��������Ʒ�������ɷֲ���ϡ���ᡢ˫��ˮ�����ᷴӦ��
��������Ʒ�������ɷֲ���ϡ���ᡢ˫��ˮ�����ᷴӦ��
��д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ���������ת�Ƶķ������Ŀ____________________��
����ͭ��˫��ˮ������ķ�Ӧ����������______________________________������2 mol��H2O2�μӷ�Ӧ�������ת�Ƶ����ʵ�����_______________��
����˫��ˮ��ϡ�����ܽ���Ʒ����ϡ�����ܽ���Ʒ��Ƚϣ����ָ��ã�Ϊʲô��____________________��
������ȡ��Ʒ������Ϊa g����������˫��ˮ�ܽ��ʣ���������ϴ�Ӹ���Ƶ�������b g��
����Ʒ�к�ͭ����������Ϊ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com