
| |||||||||||
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
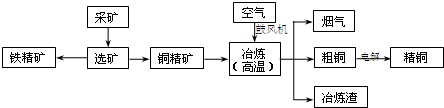
| ���� | Fe��OH��2 | Fe��OH��3 | Zn��OH��2 | Cu��OH��2 |
| Ksp | 8.0��10-16 | 4.0��10-38 | 3.0��10-17 | 2.2��10-20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
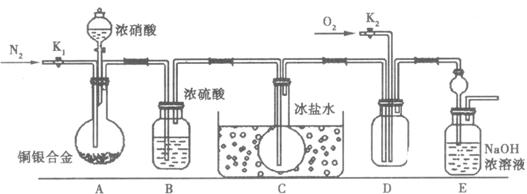
(1)ʵ�鿪ʼǰ�ȴ�A���ֵĻ���K1,����ͨһ��ʱ��ĵ����ٹر�K1����Ŀ����________________________��
(2)װ����Bƿ��������________________��
(3)A�еķ�Ӧֹͣ��D�еĻ���K2��ͨ������������Ӧȷ��NO��������D��Ӧ���ֵ�������________��ʵ�鷢�֣�ͨ��������¶ȸߵͶ�ʵ�������нϴ�Ӱ�죬Ϊ���ڹ۲�Ӧͨ��________(��䡱���ȡ�)��������
(4)Ϊ��С��������A�з�Ӧ��ɺ�D�г��������Ӧ�������еIJ�����________________________________________________________________��
(5)ʵ�����������ݣ�����ͭ���Ͻ�������15.0 g��Ũ���40 mL 13.5 mol/L��ʵ���A��Һ�����40 mL��H+Ũ�ȣ�1.0 mol/L�����跴Ӧ��������ӷ�Ҳ�ֽ⣬��
�ٲμӷ�Ӧ����������ʵ���Ϊ________�������Ѳ����Ӧ��Eװ�õ��������к���Ԫ�ص���������Ϊȷ���Ͻ���ͭ��������������ⶨ��������________________________________��
(6)��֪һ��������ͭ���Ͻ��ĩ�������Լ���������ѡ����ֻҪ��ⶨCu�����������������һ����ʵ��(ֻҪ��д����ӳʵ��ԭ���Ļ�ѧ��Ӧ����ʽ����Ҫ�ⶨ��ʵ������)��________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ��Ҧ��ѧ������ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(13��) ij�о���ʵ��С������֤Ũ��������Cu��Ag�Ͻ�ķ�Ӧ�����г�����NO2�����⣬����������NO���ɡ����ⶨCu��Ag�Ͻ���Cu����������������������ϱ������������£�NO2��N2O4��ϴ��ڣ��ڵ���0��ʱ����ֻ����ɫ��N2O4Һ�������ڡ���Ϊ�ˣ��������������ͼ��ʾ��װ�á��ش���������:
��1��д��A��Cu��HNO3��Ӧ���ܵ����ӷ���ʽ ��
��2��ʵ��ǰ�ȴ�A���ֵĻ���K1������ͨһ��ʱ��ĵ����ٹر�K1����Ŀ���� ��װ����Bƿ�������� ��
��3��ֹͣ��Ӧ��D�еĻ���K2��ͨ��O2������NO���ɣ���D�г��ֵ������� ��ʵ�鷢�֣�ͨ���O2�¶ȸߵͶ�ʵ�������нϴ�Ӱ�죬Ϊ���ڹ۲�Ӧͨ�루��ȡ����䡱�� ��O2��
��4��ʵ�����������ݣ�ʵ��ǰ��Cu��Ag�Ͻ��������15.0g��ŨHNO3��40mL 13.5 mol��L-1��ʵ���A��Һ��V��40mL c(H+)��1.0 mol��L-1�����跴Ӧ��HNO3 ���ӷ�Ҳ�ֽ⣬�ٲμӷ�Ӧ��HNO3�����ʵ���Ϊ mol
�����Ѳ����Ӧ��Eװ�õ��������к���Ԫ�ص�����m�ˣ���Ϊȷ���Ͻ���Cu��������������ⶨ�������� ������ø�����Ϊn,д���Ͻ���ͭ����������Ϊx�ˣ�������ʽ�ӣ�ֻ����ʽ�ӣ�����ⷽ�̣� ��
��5��Ϊ��С����ʵ������D�й۲쵽ʵ�����������еIJ����� ��
��6������֪Cu��Ag�Ͻ������Ϊm g�����ܽ���ȫ��ֻ����Aװ���з�Ӧ�����Һ���мIJ�����Ҳ����ȷ���Ͻ���Cu��������������ʵ���������Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(13��) ij�о���ʵ��С������֤Ũ��������Cu��Ag�Ͻ�ķ�Ӧ�����г�����NO2�����⣬����������NO���ɡ����ⶨCu��Ag�Ͻ���Cu����������������������ϱ������������£�NO2��N2O4��ϴ��ڣ��ڵ���0��ʱ����ֻ����ɫ��N2O4Һ�������ڡ���Ϊ�ˣ��������������ͼ��ʾ��װ�á��ش���������:

��1��д��A��Cu��HNO3��Ӧ���ܵ����ӷ���ʽ ��
��2��ʵ��ǰ�ȴ�A���ֵĻ���K1������ͨһ��ʱ��ĵ����ٹر�K1����Ŀ���� ��װ����Bƿ�������� ��
��3��ֹͣ��Ӧ��D�еĻ���K2��ͨ��O2������NO���ɣ���D�г��ֵ������� ��ʵ�鷢�֣�ͨ���O2�¶ȸߵͶ�ʵ�������нϴ�Ӱ�죬Ϊ���ڹ۲�Ӧͨ�루��ȡ����䡱�� ��O2��
��4��ʵ�����������ݣ�ʵ��ǰ��Cu��Ag�Ͻ��������15.0g��ŨHNO3��40mL 13.5 mol��L-1��ʵ���A��Һ��V��40mL c(H+)��1.0 mol��L-1�����跴Ӧ��HNO3 ���ӷ�Ҳ�ֽ⣬�ٲμӷ�Ӧ��HNO3�����ʵ���Ϊ mol
�����Ѳ����Ӧ��Eװ�õ��������к���Ԫ�ص�����m�ˣ���Ϊȷ���Ͻ���Cu��������������ⶨ�������� ������ø�����Ϊn,д���Ͻ���ͭ����������Ϊx�ˣ�������ʽ�ӣ�ֻ����ʽ�ӣ�����ⷽ�̣� ��
��5��Ϊ��С����ʵ������D�й۲쵽ʵ�����������еIJ����� ��
��6������֪Cu��Ag�Ͻ������Ϊm g�����ܽ���ȫ��ֻ����Aװ���з�Ӧ�����Һ���мIJ�����Ҳ����ȷ���Ͻ���Cu��������������ʵ���������Ϊ
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com