

(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ___________mol��L-1��
(2)���ı䷴Ӧ��������ij�����¦�(HI)�ı仯������(��)��ʾ���������������___________(�����������������)��
�ٺ��������£������¶� �ں��������£������¶� �ۺ��������£���С��Ӧ������� �ܺ��������£�����Ӧ������� �ݺ��¡����������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
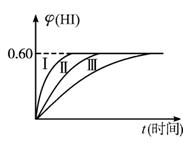
��1 mol I2(g)��2 mol H2����ij![]() 2HI(g)��H��0�����ﵽƽ�⡣HI���������w(HI)��ʱ��t�仯��ͼ����(��)��ʾ��
2HI(g)��H��0�����ﵽƽ�⡣HI���������w(HI)��ʱ��t�仯��ͼ����(��)��ʾ��

(1)�ﵽƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ______________mol��L-1��
(2)���ı䷴Ӧ�������ڼ�������w(HI)�ı仯��ͼ����(��)��ʾ������������w(HI)�ı仯��ͼ����(��)��ʾ���������������______________��������������______________��(�����������������)
�ٺ��������£������¶� �ں��������£������¶� �ۺ��������£���С��Ӧ������� �ܺ��������£�����Ӧ������� �ݺ��º��������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I2(g)+H2(g) ![]() 2HI(g);��H��0�����ﵽƽ�⡣HI����������գ�HI����ʱ��仯����ͼ�����ߣ�����ʾ��
2HI(g);��H��0�����ﵽƽ�⡣HI����������գ�HI����ʱ��仯����ͼ�����ߣ�����ʾ��

��1����ƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ_______ mol��L -1��
��2�����ı䷴Ӧ��������ij�����¦գ�HI���ı仯�����ߣ�����ʾ���������������______����������������ţ���?
�ٺ��������£������¶�?
�ں��������£������¶�?
�ۺ��������£���С��Ӧ�������?
�ܺ��������£�����Ӧ�������?
�ݺ��¡����������£������ʵ�����?
��3���������¶Ȳ��䣬����һ��ͬ��2 L�ܱ������м���a mol I2(g)��b mol H2(g)��c mol HI(g)(a��b��c������0)��������Ӧ����ƽ��ʱ��HI�����������Ϊ0.60����a��b��cӦ����Ĺ�ϵΪ_______����һ����a��b��c�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͼ1-2-9
(1)�ﵽƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ___________mol��L-1��
(2)���ı䷴Ӧ�������ڼ������¦�(HI)�ı仯��ͼ������(��)��ʾ�����������¦�(HI)�ı仯��ͼ������(��)��ʾ���������������___________��������������___________ (�����������������)��
�ٺ��������£������¶�
�ں��������½����¶�
�ۺ��������£���С��Ӧ���������
�ܺ��������£�����Ӧ���������
�ݺ��¡����������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��2 L�ܱ������м�a mol I2(g),b mol H2��c mol HI(a,b,c������0)��������Ӧ�ﵽƽ��ʱ��HI�����������Ϊ0.60,��a,b,c�Ĺ�ϵ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�껦�ư���л�ѧ6.1��ʾ��ѧ��Ӧ���ʺ�ƽ��֮����ϰ���������棩 ���ͣ������
��1 mol I2(g) ��2 mol H2����2L�ܱ������У���һ���¶��·�����Ӧ��
I2(g) + H2(g) 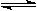 2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ
2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ
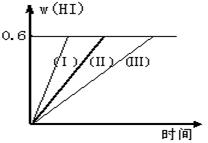
��1����ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ д���÷�Ӧ��ƽ�ⳣ������ʽ _____________________________________��
��2�����ı䷴Ӧ�������ڼ�������w(HI)�ı仯������(��) ��ʾ������������w(HI)�ı仯������(��) ��ʾ��������������� ���������������� ��
������������������ţ�
�ٺ��������£������¶ȣ� �ں��������£������¶ȣ��ۺ��������£���С��Ӧ��������� �ܺ��������£�����Ӧ��������� �ݺ��º��������£������ʵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣���1 mol I2(g) ��2 mol H2����2L�ܱ������У���һ���¶��·�����Ӧ�� H2(g) + I2(g)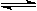 2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��

��1����ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ ��
��2�����ı䷴Ӧ�������ڼ�������w(HI)�ı仯������(��) ��ʾ������������w(HI)�ı仯������(��) ��ʾ��������������� ���������������� ��
������������������ţ�[��Դ:ѧ#��#��Z#X#X#K]
�ٺ��������£������¶ȣ��ں��������£������¶ȣ��ۺ��������£���С��Ӧ����������ܺ��������£�����Ӧ����������ݺ��º��������£������ʵ�������
��3���������¶Ȳ��䣬����һ����ͬ��2L�ܱ������м���a mol I2(g)��b mol H2(g)��c mol HI��a��b��c������0����������Ӧ����ƽ��ʱ��HI�����������Ϊ0.6����a��b��c�Ĺ�ϵ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com