
A�������µ��볣��ΪKa����HA ��Һ�� c ��H+��=
| ||
| B��0.2mol?L-1 CH3COOH��Һ��0.1mol?L-1NaOH��Һ��������2c��H+��-2c��OH-��=c��CH3COO-��-c��CH3COOH�� | ||
| C��������Na2SO4��Һ���뵽����ʯ��ˮ�У��а�ɫ����������˵��Ksp[Ca��OH��2]����Ksp��CaSO4�� | ||
| D�������£���0.1mol/L NH4HSO4��Һ�еμ�NaOH��Һ������c��Na+����c��NH4+����c��SO42-����c��OH-��=c��H+�� |
| Ka |
| Ka |
| Ka |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Al2��SO4��3��Һ���ɡ����������أ�����ʣ�������Al2O3 |
| B����ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ���������һ����SO2 |
| C��ȡ����ij��Һ���μ�����KSCN��Һ����������������ȡ����ԭ��Һ���ȵ�����������ˮ���ٵμ�����KSCN��Һ����Һ��Ѫ��ɫ����ԭ��Һ��һ����Fe2+ |
| D���ڵ�����Һ�м���ϡ���Ტ����Ƭ�̺��ټ�������������Һ��ˮԡ���Ⱥ�û���������ɣ�˵������û��ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
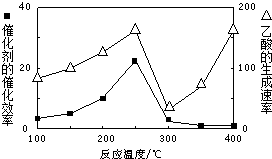 CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�꣮
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�꣮| �¶� | 200�� | 250�� | 300�� |
| K | 56 | 64 | 80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����H1����H2 |
| B����H1=��H2 |
| C����H1����H2 |
| D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���٢� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��n+11 | B��n+1 |
| C��n-6 | D��n-5 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com