
| 256.65kJ |
| 0.4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״���£�22.4LCl2��HCl�Ļ������������������Ϊ2 NA |
| B����״���£�11.2L �Ҵ������ķ�����Ϊ0.5NA |
| C��0.1molCH4�����ĵ�����һ��ΪNA |
| D��22.4 L N2�������ķ�����һ��ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϡ���������ɫ��ζ���壬������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�ԭ��Һ���ܺ�CO32- |
| B������BaCl2��Һ�а�ɫ�����������ټ����ᣬ������ʧ��ԭ��Һһ������SO42- |
| C������AgNO3��Һ�а�ɫ����������ԭ��Һһ������Cl- |
| D������Na2CO3��Һ�а�ɫ�����������ټ����ᣬ��ɫ������ʧ��ԭ��Һ��һ������Ba2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ˮ������轺�������� |
| B��Ư��Һ��Ư�۾�����Ҫ�ɷ־�Ϊ���� |
| C��NO2��SO3���������������� |
| D�����ᡢһˮ�ϰ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
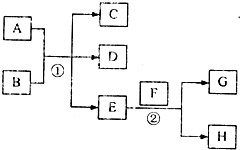 ��֪������EΪ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ���壨��Ӧ��������ʡ�ԣ�
��֪������EΪ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ���壨��Ӧ��������ʡ�ԣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ��/h | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| N2 | 1.500 | 1.400 | 1.200 | a | a | 0.900 | |
| H2 | 4.500 | 4.200 | 3.600 | b | b | 2.700 | 2.100 |
| NH3 | 0 | 0.200 | 0.600 | c | c | 0.200 | 0.600 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com