
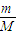 ������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������
������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������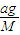 ��
��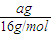 =4��11��
=4��11��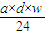 mol��
mol��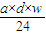 mol=
mol=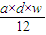 mol��
mol��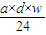 mol��
mol��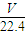 mol������Ϊ
mol������Ϊ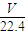 mol×17g/mol=
mol×17g/mol=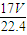 g��
g��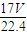 g+1000g�������V=
g+1000g�������V=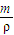 =
=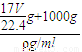 =
=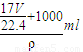 ����
����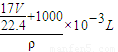 ��
��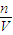 =
=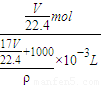 =
=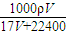 mol/L��
mol/L��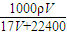 mol/L��
mol/L��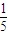 mol��
mol�� mol��
mol��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a��d��w |
| 12 |
| a��d��w |
| 12 |
| 1000��V |
| 17V+22400 |
| 1000��V |
| 17V+22400 |
| 1 |
| 5 |
| 1 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ����һ�и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��8�֣���1������ͬ�ݻ����ܷ�����A��B�������£�A�г���a g A���壬B�г���a g CH4���壬A��B�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ_________________��
��2��ij�Ȼ�þ��Һ���ܶ�Ϊd g/cm3������þ���ӵ���������Ϊw��a mL����Һ��Cl-
�����ʵ���Ϊ ��
��3����״����VL�����ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1g/ mL����������Һ���ܶ�Ϊ�� g/ mL, �����Һ�����ʵ����ʵ���Ũ��Ϊ ��
��4���� 11P + 15 CuSO4 +24 H2O =" 5" Cu3P +6 H3PO4 +15 H2SO4�У�ÿ 1 mol CuSO4�������������ʵ����� __________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��8�֣���1������ͬ�ݻ����ܷ�����A��B�������£�A�г���a g A���壬B�г���a g CH4���壬A��B�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ_________________��
��2��ij�Ȼ�þ��Һ���ܶ�Ϊd g/cm3������þ���ӵ���������Ϊw��a mL����Һ��Cl-
�����ʵ���Ϊ ��
��3����״����VL�����ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1g/ mL����������Һ���ܶ�Ϊ�� g/ mL, �����Һ�����ʵ����ʵ���Ũ��Ϊ ��
��4���� 11P + 15 CuSO4 +24 H2O = 5 Cu3P +6 H3PO4 +15 H2SO4 �У�ÿ 1 mol CuSO4�������������ʵ����� __________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com