
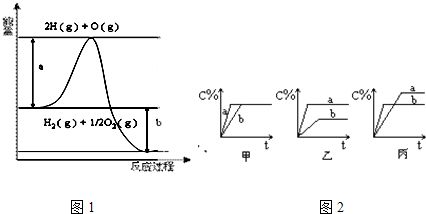



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
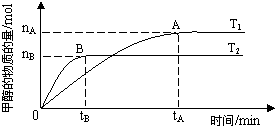 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��| nA |
| tA |
| nA |
| tA |
| a |
| 2 |
| a |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����ʵ����и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��8�֣���1����֪H2��g��ȼ����Ϊ-285��8kJ��mol-1��̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
��2����֪0.4 molҺ̬��(N2 H4)������H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65 kJ��������д���ø÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________________��
����֪ H2O��l��=== H2O��g����H����44kJ��mol��1��16gҺ̬��������H2O2��Ӧ�����ɵ�����Һ̬ˮ�ų���������_____________ kJ��
������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣���1����֪H2��g��ȼ����Ϊ-285��8kJ��mol-1��̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
��2����֪0.4 molҺ̬��(N2 H4)������H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65 kJ��������д���ø÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________________��
����֪ H2O��l��=== H2O��g����H����44kJ��mol��1��16gҺ̬��������H2O2��Ӧ�����ɵ�����Һ̬ˮ�ų���������_____________ kJ��
������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ������ԭƽһ�и߶����ϣ����л�ѧ�Ծ�����ͨ�ࣩ�������棩 ���ͣ������
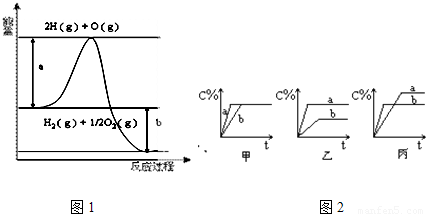
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com