
(8��) ��һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g)+H2(g) CO(g)+H2O(g),�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���£�
CO(g)+H2O(g),�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���£�
|
t�� |
700 |
800 |
830 |
1000 |
1200 |
|
K |
0.6 |
0.9 |
1.0 |
1.7 |
2.6 |
��ش��������⣺��1���÷�Ӧ�Ļ�ѧƽ�ⳣ��K = ��
��2���÷�ӦΪ ��Ӧ��������ȡ����ȡ���
��3��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c��CO2����c��H2����c��CO����c��H2O�������жϴ�ʱ���¶�Ϊ �档
(4)830��,��1 L�Ĺ̶��������ܱ������з���2 mol CO2��1 mol H2,ƽ���CO2��ת����Ϊ ,
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012������˲���ѧУ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
(8��)��һ��������ܱ������У��������»�ѧ��Ӧ��A(g)��B(g)  C(g)��D(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±����ش��������⣺
C(g)��D(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±����ش��������⣺
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡʵ����ѧ��У����12���¿���ѧ�Ծ����������� ���ͣ������
(8��)��һ��������ܱ������У��������»�ѧ��Ӧ��
CO2��g����H2��g�� CO��g����H2O��g����
CO��g����H2O��g����
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ�������и���12���¿���ѧ�Ծ� ���ͣ�ʵ����
.(14��) ��������ƣ�Na2S2O3���dz��õĻ�ԭ������ά����C����ѧʽC6��8��6����ˮ��Һ�м������I2��Һ��ʹά������ȫ������ʣ���I2��Na2S2O3����Һ�ζ����ɲⶨ��Һ��ά����C�ĺ����������ķ�ӦΪ��
C6H8O6+I2===C6H6O6+2H++2I- 2S2O32-+I2===S4O62-+2I-
��һ�������ijά����C��Һ�м���1.2molL-1 I2��Һ25ml,��ַ�Ӧ����1.0molL-1Na2S2O3����Һ�ζ�ʣ���I2����ش��������⣺
��1������100mL 1.0molL-1 Na2S2O3����Һ��ʹ�õ���������ƽ��ҩ�ס���ͷ�ι��⣬����Ҫ___________________________________������ʱ���ý�ͷ�ιܼ�ˮ�������̶��߲�����ƿ�������һ��������_______________________________________
��2���ζ�ʱ����ѡ��_________________��ָʾ�����ζ�ʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��_____________________________________��ֱ������________________________________________����Ϊֹ��
��3�����ζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ����Ӧ����Na2S2O3����Һ�������_______________���Ը��ݸ�ʵ�����ݼ������Һ��ά����C�����ʵ����� mol��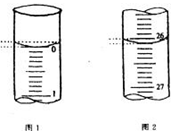
��4���ж����в����Ը���Һ��ά����C�����ʵ����ⶨ�����Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�������Ʊ���Һ�����У��ձ��е�Na2S2O3��Һ������������ʹ�ⶨ���_______��
���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬ʹ�ⶨ���_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��У����12���¿���ѧ�Ծ��������棩 ���ͣ������
(8��)��һ��������ܱ������У��������»�ѧ��Ӧ��
CO2��g����H2��g�� CO��g����H2O��g����
CO��g����H2O��g����
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
|
t�� |
700 |
800 |
830 |
1000 |
1200 |
|
K |
0.6 |
0.9 |
1.0 |
1.7 |
2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK�� ��
��2���÷�ӦΪ ��Ӧ��ѡ�����ȡ����ȣ���
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬�������� ��
a��������ѹǿ���� b����������� c��CO������
c��v����H2����v����H2O�� d��c��CO2����c��CO��
��4��ij�¶��£�ƽ��Ũ�ȷ�����ʽ�� c��CO2����c��H2����c��CO����c��H2O�������жϴ�ʱ���¶�Ϊ �档
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����12���¿���ѧ�Ծ� ���ͣ�ʵ����
.(14��) ��������ƣ�Na2S2O3���dz��õĻ�ԭ������ά����C����ѧʽC6��8��6����ˮ��Һ�м������I2��Һ��ʹά������ȫ������ʣ���I2��Na2S2O3����Һ�ζ����ɲⶨ��Һ��ά����C�ĺ����������ķ�ӦΪ��
C6H8O6+I2===C6H6O6+2H++2I- 2S2O32-+I2===S4O62-+2I-
��һ�������ijά����C��Һ�м���1.2molL-1 I2��Һ25ml,��ַ�Ӧ����1.0molL-1Na2S2O3����Һ�ζ�ʣ���I2����ش��������⣺
��1������100mL 1.0molL-1 Na2S2O3����Һ��ʹ�õ���������ƽ��ҩ�ס���ͷ�ι��⣬����Ҫ___________________________________������ʱ���ý�ͷ�ιܼ�ˮ�������̶��߲�����ƿ�������һ��������_______________________________________
��2���ζ�ʱ����ѡ��_________________��ָʾ�����ζ�ʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��_____________________________________��ֱ������________________________________________����Ϊֹ��
��3�����ζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ����Ӧ����Na2S2O3����Һ�������_______________���Ը��ݸ�ʵ�����ݼ������Һ��ά����C�����ʵ����� mol��

��4���ж����в����Ը���Һ��ά����C�����ʵ����ⶨ�����Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�������Ʊ���Һ�����У��ձ��е�Na2S2O3��Һ������������ʹ�ⶨ���_______��
���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬ʹ�ⶨ���_________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com