
����Ŀ��һ���¶��£������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g)![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
������� | �¶�(��) | ��ʼ���ʵ���(mol) | ƽ�����ʵ���(mol) | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | 387 | 0.20 | 0.080 | 0.080 |
�� | 387 | 0.40 | ||
�� | 207 | 0.20 | 0.090 | 0.090 |
A.�÷�Ӧ������ӦΪ���ȷ�Ӧ
B.��ƽ��ʱ����������CH3OCH3��Ũ�ȴ���0.16 mol/L
C.��ƽ��ʱ����������![]() ���������еĴ�
���������еĴ�
D.����ʼʱ���������г���CH3OH(g)0.30 mol��CH3OCH3(g)1.50 mol��H2O(g)0.30 mol����Ӧ�����淴Ӧ�������
���𰸡�AD
��������
A���Ա�I�����֪�������¶�CH3OCH3(g)�����ʵ�����С��˵�������¶ȣ�ƽ�������ƶ����������¶ȣ���ѧƽ�������ȷ�Ӧ�ƶ����ʸ÷�Ӧ������ӦΪ���ȷ�Ӧ��A��ȷ��
B�����ЧΪ����ƽ�������ѹǿ����һ�����÷�Ӧ�������������ķ�Ӧ������ѹǿ��ѧƽ�ⲻ�ƶ�������������е�CH3OH����������������е���ȡ�I��ƽ��ʱCH3OCH3�����ʵ�����0.080 mol������������CH3OCH3�����ʵ���Ϊn(CH3OCH3)=2��0.080 mol=0.16 mol����Ӧ������1 L����II�ﵽƽ��ʱCH3OCH3�����ʵ���Ũ��Ϊc(CH3OCH3)=![]() =0.16 mol/L��B����
=0.16 mol/L��B����
C��II�дﵽƽ��ʱ��c(CH3OCH3)=![]() =0.16 mol/L��c(CH3OH)=
=0.16 mol/L��c(CH3OH)=![]() =0.08 mol/L��
=0.08 mol/L��![]() =0.5��III�дﵽƽ��ʱ��c(CH3OCH3)=
=0.5��III�дﵽƽ��ʱ��c(CH3OCH3)=![]() =0.090 mol/L��c(CH3OH)=
=0.090 mol/L��c(CH3OH)=![]() =0.020 mol/L��
=0.020 mol/L��![]() =0.222��0.25�����Դ�ƽ��ʱ����������
=0.222��0.25�����Դ�ƽ��ʱ����������![]() ���������е�С��C����
���������е�С��C����
D����������ƽ��ʱc(CH3OCH3)=c(H2O)=![]() =0.080 mol/L��c(CH3OH)=
=0.080 mol/L��c(CH3OH)=![]() =0.04 mol/L���������л�ѧƽ�ⳣ��K1=
=0.04 mol/L���������л�ѧƽ�ⳣ��K1=![]() =4������ʼʱ���������г���CH3OH(g)0.30 mol��CH3OCH3(g)1.50 mol��H2O(g)0.30 mol�������������ݻ���1 L�������ʵ�Ũ���������ʵ�������ֵ����ȣ���ʱŨ����Qc=
=4������ʼʱ���������г���CH3OH(g)0.30 mol��CH3OCH3(g)1.50 mol��H2O(g)0.30 mol�������������ݻ���1 L�������ʵ�Ũ���������ʵ�������ֵ����ȣ���ʱŨ����Qc=![]() =5��4=K����Ӧ�����淴Ӧ������У�D��ȷ��
=5��4=K����Ӧ�����淴Ӧ������У�D��ȷ��
�ʺ���ѡ����AD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() ��
��![]() �����Ʊ�����
�����Ʊ�����![]() ��
��![]() ����Һ��������Һ�����ڼ���ȩ����Ҳ�����ں������Ƿ�Ӧ�Ʊ�����
����Һ��������Һ�����ڼ���ȩ����Ҳ�����ں������Ƿ�Ӧ�Ʊ�����![]() ��
��
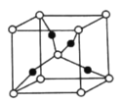
(1)��̬![]() �ĺ�������Ų�ʽΪ__________��
�ĺ�������Ų�ʽΪ__________��
(2)��![]() ��Ϊ�ȵ������һ�ַ���Ϊ________���ѧʽ����
��Ϊ�ȵ������һ�ַ���Ϊ________���ѧʽ����
(3)![]() ��
��![]() ��Ӧ������
��Ӧ������![]() ��
��![]() �е���λԭ��Ϊ________����Ԫ�ط��ţ���
�е���λԭ��Ϊ________����Ԫ�ط��ţ���
(4)��ȩ(![]() )��̼ԭ�ӵĹ���ӻ�������_________��
)��̼ԭ�ӵĹ���ӻ�������_________��![]() �к���
�к���![]() ������ĿΪ________
������ĿΪ________![]() ��
��
(5)һ��![]() ����(��ͼ)�У�
����(��ͼ)�У�![]() ԭ�ӵ���ĿΪ________��
ԭ�ӵ���ĿΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������﮵��Ӧ�ù㷺�� ��طŵ��������Ҫ����Li4Ti5O12�� ����������Fe�� ��ͨ�����й������̻����ѡ� ﮡ��ش��������⣺

(1) Li4Ti5O12�У� TiԪ�صĻ��ϼ�Ϊ___�� ��Һ1�к���������������___(�����ӷ���)��
(2) ���ʱ Li4Ti5O12�����ķ�Ӧ�ǣ� Li4Ti5O12+7H2SO4+5H2O2��2Li2SO4+5[TiO(H2O2)]SO4+7H2O�� �÷�Ӧ�Ƿ�����������ԭ��Ӧ��___(�� ������ �� ������)�� ����1��___��
(3) ��[TiO(H2O2)]SO4��Һ�м���Na2SO3��Һ������Ӧ�����ӷ���ʽΪ___��
(4) ��TiOSO4��Һ��ͨ��NH3������Ӧ�����ӷ���ʽΪ___��
(5) ����TiO(OH)2������ˮϴ�ӵ�Ŀ����___��
(6) ��ȡʱ�� �¶ȶ���ȡ�ʵ�Ӱ����ͼ��ʾ����ͼ����֪ʵ��ʱѡ���ڳ����½��м��ɣ� ������__��

(7)����ȡ�� �� ������ȡ�� �ɼ�ʾΪ��[TiO(H2O2)]2+��2(HA)2![]() [TiO(H2O2)](HA2)2��2H+������ȡ�����м�����Լ�X��___��
[TiO(H2O2)](HA2)2��2H+������ȡ�����м�����Լ�X��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС������������ȡ������ʯ�������ܼ��ӻ���������ȡ��֬��װ����ͼ��ʯ���ѵ���Ҫ�ɷ�Ϊ���������Ļ����г�Ϊ��30��~60�档����˵���������
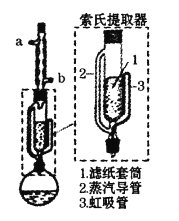
A.ʯ�����ӷ���ȼ�գ���ȡ���̲���ѡ������ֱ�Ӽ���
B.ʯ���ѵķг̽ϴ���ǰ���ؼ����ʯ
C.��ƿ�е�ʯ�������Ⱦ���ܽ��������ܣ���ȴ�������ֽ��Ͳ���黨���Ӵ�������ȡ
D.������ȡ��ʹ���ܼ��٣���ѭ��������ȡ����ȡЧ�ʸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na3OCl��һ�����õ����ӵ��壬���з����ѿ���ṹ���ش��������⣺
(1)��̬Tiԭ��4s����ϵ�һ�����Ӽ�����4p������γɼ���̬��д���ü���̬�۲�����Ų�ʽ_____________
(2)п����λ��ͬ���壬��п��ͭ���ڡ�����4��ͭ��пԪ�ص���Ӧ״̬����п��[Ar]3d104s2����п��[Ar]3d104s1����ͭ��[Ar]3d104s1����ͭ��[Ar]3d10��ʧȥ1��������Ҫ�������ɴ�С������_________(����ĸ)��
A.�ܢڢ٢� B.�ܢڢۢ� C.�٢ڢܢ� D.�٢ܢۢ�
(3)��O��ClԪ�ؿ���ɲ�ͬ�ĵ��ʺͻ��������Cl2O2���ƻ������㡣
��Cl2O2�ķе��H2O2�ͣ�ԭ����____��
��O3���ӵ�����ԭ���ӻ�����Ϊ______����O3��Ϊ�ȵ��������______(����дһ��)��
(4)Na3OCl�������·����Ƶã�2Na+2NaOH+2NaCl 2Na3OCl+H2�����ڸ÷�Ӧ�У��γɵĻ�ѧ����_____(����)��
2Na3OCl+H2�����ڸ÷�Ӧ�У��γɵĻ�ѧ����_____(����)��
A.������ B.���Ӽ� C.��λ�� D.���Լ� E.�Ǽ��Լ�
(5)Na3OCl��������������ϵ���侧���ṹ��ͼ��ʾ����֪����������Ϊanm���ܶ�Ϊdg��cm3��
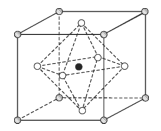
��Na3OCl�����У�Oλ�ڸ�����λ�ã�Clλ��______λ�á�
����a��d��ʾ�����ӵ�������ֵNA=_____(�м���ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��W��X��Y��ZΪԭ���������μ�С�Ķ���������Ԫ�أ���֪W��Y��Z������������֮�͵���X��������������������Ԫ���γ�ij������Ľṹ��ͼ��ʾ��������������ȷ����( )
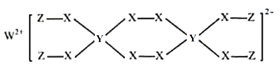
A.���⻯����ȶ��ԣ�X��YB.�����Ӱ뾶��X��W
C.Y������������Ӧˮ����Ϊ����D.�û������и�Ԫ�ؾ�����8�����ȶ��ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������![]() ��
��![]() �Ʊ�
�Ʊ�![]() ��ԭ��Ϊ��
��ԭ��Ϊ��
��![]()
��![]()
��֪��A��B��Ϊ�л��������Ӧ�����Է����У�NA���������ӵ�����������˵����ȷ����
A.BΪ![]() ��
��![]() �Ʊ�
�Ʊ�![]() �Ĵ���
�Ĵ���
B.1mol![]() ��������B��һ�������·�����Ӧ�ڣ���ת��2NA������
��������B��һ�������·�����Ӧ�ڣ���ת��2NA������
C.��Ӧ�ٵķ�Ӧ������������ڲ����������
D.��״���£�22.4L![]() ��NA������
��NA������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�о�����п���Ȼ�ͭ��Һ֮�䷴Ӧ����ʵ���и�ͬѧ�۲쵽�������У�
i.�����������ݣ�ii.�к�ɫ�������ɣ�iii.��Һ�в�����ɫ������
Ϊ�˽�����������ijͬѧ�������ϣ����������Ϣ��
��� | ��ѧ��Ӧ���ӷ���ʽ |
1 | Zn+Cu2+ |
2 | Zn+2Cu2+ |
3 | Cu++2Cl- |
4 | Cu++Cl- |
��1���������ӷ���ʽ���Ͳ����������ݵ�ԭ��___��
��2��Zn��CuCl2��Ӧ���ɰ�ɫ���������ӷ���ʽ��___��
��3��Ϊ��̽��Ӱ�����ɰ�ɫ���������أ���ͬѧ��һ��ʵ�顣ȡ��ͬŨ��CuCl2��Һ������п���������̲����������ݺͺ�ɫ���壬����ʵ���������¡�
| ��� | Ũ��(rnol/L) | �Լ���п�������� | ʵ������ |
a | 0.5 | пƬ | ���̳���������ɫ���� | |
b | 1 | пƬ | ���̳��ְ�ɫ���� | |
1 | п�� | ���̳��ִ�����ɫ���� | ||
d | 1 | пƬ������NaCl���� | ������ɫ��������Ѹ���ܽ� |
�ٶԱ�ʵ��a��b��ʵ�������___��
��ijͬѧ�ӻ�ѧƽ��ĽǶȷ�����d�а�ɫ�����ܽ���ܵ�ԭ����___�������ӷ���ʽ��ʾ����Ϊ֤����ͬѧ������ԭ����ȷ����b�Թ��м�������___�����۲쵽___��֤����ͬѧ������ԭ����ȷ��
��4�����ó�������ȥ������ʵ��������Ӧ�ù㷺��
���ڹ�ҵ��ұ��п��Ϊ�˳�ȥZnSO4���Һ��Һ�е�C1-���ɼ���___��___�����ɳ�������ȥ��
��ͨ����һ���������ϵ�֪��CuCl���γ�����Һ��pH��Cu2+������Ũ���йأ�һ�������£�ͨ��ʵ��ó�pH��Cu2+Ũ�ȶ�Cl-������Ӱ����ͼ��ʾ��
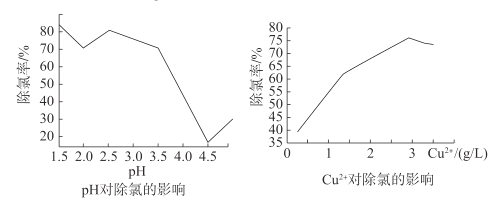
����ͼ����ȥ�õ��Һ��Cl-�����������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ж���ȷ����(����)
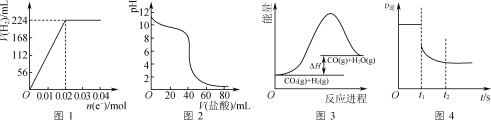
A.ͼ1�ɱ�ʾ���200 mL 0.1 mol��L��1 NaCl��Һ�����У������������(��״��)��ת�Ƶ������ʵ����Ĺ�ϵ����
B.ͼ2�ɱ�ʾ������0.1 mol��L��1����μӵ�40 mL 0.1 mol��L��1 NaOH��Һ�ĵζ�����
C.���������Է����еķ�ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)�������仯��ͼ3��ʾ����÷�Ӧ����S>0
CO(g)��H2O(g)�������仯��ͼ3��ʾ����÷�Ӧ����S>0
D.ͼ4�ɱ�ʾ��ӦN2(g)��3H2(g)![]() 2NH3(g)��t1ʱ�������������ʱ��v����ʱ��ı仯����
2NH3(g)��t1ʱ�������������ʱ��v����ʱ��ı仯����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com