
| ʵ���� | HA�����ʵ��� Ũ�ȣ�mol•L-1�� | NaOH�����ʵ��� Ũ�ȣ�mol•L-1�� | ��Ϻ� ��Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
���� ��1�������ʵ������ʱ������ǡ�÷�Ӧ�����Σ�������Һ��pH�ж�����ǿ����
��2�������ʱ�������������Һ�����ԣ�˵����Ϊ���ᣬ��Һ������ΪHA��NaA��
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��ϵ���غ��жϣ�
��4���ɵ���غ��ϵʽ���ε�c��Na+��-c��A-��=c��OH-��-c��H+����
��5�������ʵ�����Ӧ����NaA����Һ�Լ��ԣ���NaA����ˮ�⣮
��� �⣺��1����HA��ǿ�ᣬǡ����NaOH��Һ��Ӧ����ǿ��ǿ���Σ�pH=7����HA�����ᣬ���ɵ�NaAˮ���Լ��ԣ�pH��7�����Բ����ж�HA������ǿ����
�ʴ�Ϊ�����ܣ�
��2�������ʱ�������������Һ�����ԣ�˵����Ϊ���ᣬ��Һ������ΪHA��NaA����Һ�д���HA�ĵ���ƽ�⣬NaA��ˮ��ƽ�⣬ˮ�ĵ���ƽ�⣻
�ʴ�Ϊ��3��
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH��7��˵��A-��ˮ�����HA�ĵ��룬��������Ũ���ɴ�С��˳��Ϊc��Na+����c��A-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��A-����c��OH-����c��H+����
��4���ɵ���غ��ϵʽ���ε�c��Na+��-c��A-��=c��OH-��-c��H+��=��10-4-10-10��mol•L-1��
�ʴ�Ϊ��10-4-10-10��
��5�������ʵ�����Ӧ����NaA����Һ�Լ��ԣ���NaA����ˮ�⣬��ˮ�ⷽ��ʽΪ��A-+H2O?HA+OH-��
�ʴ�Ϊ��A-+H2O?HA+OH-��
���� ���⿼����������ʵĵ��롢����Ũ�ȴ�С�ıȽϣ���Ŀ�Ѷ��еȣ���ȷ������ʵ����ص��������غ㡢����غ�������غ�����������𣬲����ڿ���ѧ���ķ��������ͶԻ���֪ʶ��Ӧ��������


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��9 | B�� | 9��1 | C�� | 10��1 | D�� | 1��10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ���ż� | B�� |  �в� | C�� |  ���� | D�� |  ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
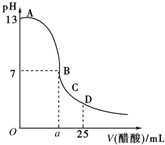 25��ʱ����25mL 0.1mol/L��NaOH��Һ�У���μ���0.2mol/L��CH3COOH��Һ����Һ��pH�仯����ͼ��ʾ�����з����Ľ�������ȷ���ǣ�������
25��ʱ����25mL 0.1mol/L��NaOH��Һ�У���μ���0.2mol/L��CH3COOH��Һ����Һ��pH�仯����ͼ��ʾ�����з����Ľ�������ȷ���ǣ�������| A�� | B��ĺ�����a=12.5 | |
| B�� | C��ʱ��Һ���У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | D��ʱ��Һ���У�c��CH3COO-��+c��CH3COOH��=2c��Na+�� | |
| D�� | ������A��B�������һ�㣬��Һ�ж��У�c��Na+����c��CH3COO-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
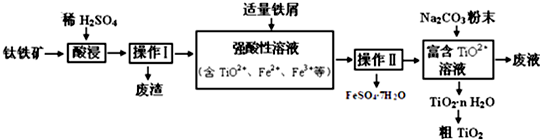
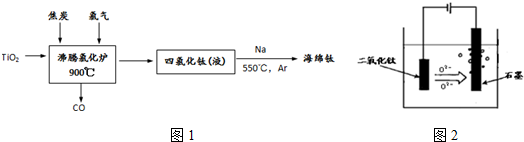
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
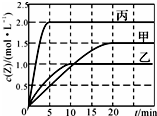 ��ס��ҡ��������ݻ��̶�������ܱ������г���һ������X��Y��һ�������·�����Ӧ X��g��+aY��g��?2Z��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������Z��Ũ����ʱ��仯�ֱ�����ͼ���±�����ʾ������˵������ȷ���ǣ�������
��ס��ҡ��������ݻ��̶�������ܱ������г���һ������X��Y��һ�������·�����Ӧ X��g��+aY��g��?2Z��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������Z��Ũ����ʱ��仯�ֱ�����ͼ���±�����ʾ������˵������ȷ���ǣ������� | ���� | �� | �� | �� | |
| �ݻ�/L | 0.5 | 0.5 | 1.0 | |
| �¶�/�� | T1 | T2 | T2 | |
| ��Ӧ����ʼ�� | 1.0 mol X 0.5 mol Y | 1.0 mol X 0.5 mol Y | 4.0 mol X 2.0 mol Y |
| A�� | �÷�Ӧ�¶����ߣ�ƽ�ⳣ����С | |
| B�� | 20 min�ڼ������з�Ӧ��ƽ�����ʣ�v��X��=0.0375mol•��L•min��-1 | |
| C�� | 10 minʱ�������������䣬�����������ټ���2 mol Z��ƽ�����淴Ӧ�����ƶ� | |
| D�� | �����������䣬����С�ҵ������Y��ת�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com