
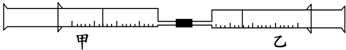
| ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ ������ |
| 1 | 10mL FeSO4��Һ | 10mL NH3 | ���ɰ�ɫ���������ɫ |
| 2 | 30mL NO2 | 10mL H2O��l�� | ʣ��ɫ���� |
| 3 | 15mL Cl2 | 40mL NH3 |
���� ��1��+2�۵��������ױ�����������+3�۵������ӣ�NH3��FeSO4��Һ��ˮ��Ӧ���ɰ�ˮ����ˮ�����OH-��FeSO4��Һ��+2�۵������ӷ�Ӧ����Fe��OH��2��Fe��OH��2�������е�����������
��2������N2O4��ת��ΪNO2���Լ�NO2��ˮ��Ӧ���������һ��������������
��3����������ɫΪ����ɫ��3Cl2+2NH3�TN2+6HCl������İ���������Ȼ��ⷴӦ������
��� �⣺��1����������ˮ��NH3+H2O?NH3•H2O��FeSO4+2NH3•H2O�TFe��OH��2��+��NH4��2SO4�����ӷ���ʽΪFe2++2NH3��H2O=Fe��OH��2��+2NH4+��Fe��OH��2Ϊ��ɫ�������ڿ������ױ������е�����������������Ӧ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��Fe��OH��3Ϊ���ɫ��
�ʴ�Ϊ�����ɫ��Fe2++2NH3��H2O=Fe��OH��2��+2NH4+��
��2��������30mL������NO2��N2O4�Ļ�����壬��Ҫ�Ƕ����������壬����10mLH2O��l������3NO2+H2O=2HNO3+NO��֪����10mLNO���������ʣ�����ɫ������һ��������NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ��3NO2+H2O=2HNO3+NO��
�ʴ�Ϊ��NO��3NO2+H2O�T2HNO3+NO��
��3��15molCl2��40mLNH3����Ϸ�����Ӧ
���ݷ���ʽ��֪��3Cl2+2NH3 �TN2 +6HCl��
3 2 1 6
15mol 10mol 5mol 30mol
ʣ�ఱ�����ʵ���Ϊ40mol-10mol=30mol��
�� NH3+HCl�TNH4Cl
30mol 30mol
��֪�����ɵ�HCl��ʣ���NH3��Ӧ����NH4Cl���Ȼ���Ͱ���ǡ�÷�Ӧ��Cl2����������ȫ��Ӧ���������ʣ�������Ϊ���������ʵ���Ϊ5mol��
�ʴ�Ϊ������ɫ��Ϊ��ɫ��5��
���� ���⿼����Ԫ�ؼ��仯�����֪ʶ��Ϊ�߿��μ����ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬�漰�����ȵĻ���������ʣ��ۺ��Խ�ǿ����ѧϰ�ý�֪ʶʱ������صķ�Ӧ����ʽ����Ŀ�Ѷ��е�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� |  ����������Ҵ� | B�� |  ����пͭԭ��� | C�� |  �Ʊ��������� | D�� |  �Ʊ�����ˮ �Ʊ�����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
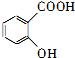
 ��Ϊ���о�X�Ľṹ����������A��һ��������ˮ��ֻ�õ�B������ʽΪC8H8O3����C������ʽΪC7H 6O3����C��FeCl3ˮ��Һ����ɫ����NaHCO3��Һ��Ӧ��CO2������
��Ϊ���о�X�Ľṹ����������A��һ��������ˮ��ֻ�õ�B������ʽΪC8H8O3����C������ʽΪC7H 6O3����C��FeCl3ˮ��Һ����ɫ����NaHCO3��Һ��Ӧ��CO2������
 ��
�� ��
�� ��Ϊ��Ҫԭ��
��Ϊ��Ҫԭ�� �Ʊ��ϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$H2C=CH2$\stackrel{Br_{2}}{��}$
�Ʊ��ϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$H2C=CH2$\stackrel{Br_{2}}{��}$ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڳ��³�ѹ�£�11.2 LN2���еķ�����Ϊ0.5 NA | |
| B�� | �ڳ��³�ѹ�£�1molNe���е�ԭ����ΪNA | |
| C�� | 71gCl2����ԭ����ΪNA | |
| D�� | ��ͬ�¡�ͬѹ�£���ͬ������κ����嵥��������ԭ������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ���������������H3+��H3������Ϊͬλ�� | |
| B�� | ��ɫ��Ӧʵ����������У�û�в�˿��Ҳ�����ù���������˿���� | |
| C�� | N2�ĽṹʽΪN��N������ʽΪ | |
| D�� | Na+�Ľṹʾ��ͼ  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2�ǰ�ɫ���壬����ˮ���÷ų������������������� | |
| B�� | ��Na2O2��CO2�ķ�Ӧ�У���������Na2O2����ԭ����CO2 | |
| C�� | Na2O���ȶ����ܼ���������������Na2O2 | |
| D�� | Na2O2����ˮ��Ӧ������NaOH������Na2O2�Ǽ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2��I2��HI��Ũ��֮��1��1��2 | |
| B�� | H2��I2��HI��Ũ�ȱ��ֲ��� | |
| C�� | ���������ܶȱ��ֲ��� | |
| D�� | �������������ѹǿ����ʱ��仯���仯 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com