
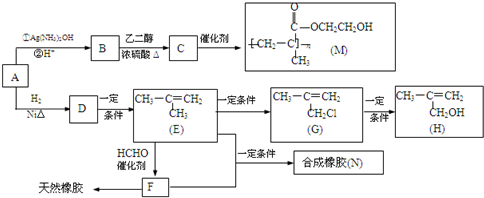
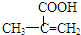 ���ò��������ж���ͬ���칹�壬��д�����������Һ���̼̼˫����ͬ���칹��Ľṹ��ʽ
���ò��������ж���ͬ���칹�壬��д�����������Һ���̼̼˫����ͬ���칹��Ľṹ��ʽ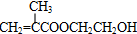 ��B���Ҷ�����Ӧ����C����B�Ľṹ��ʽΪ��
��B���Ҷ�����Ӧ����C����B�Ľṹ��ʽΪ��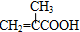 ��Aͨ����������Ӧ������H+��Ӧ����B����A�Ľṹ��ʽΪ��
��Aͨ����������Ӧ������H+��Ӧ����B����A�Ľṹ��ʽΪ��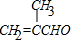 ��
��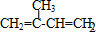 �����ݼӾ۷�Ӧԭ������д��E��F��Ӧ�Ļ�ѧ����ʽΪ��
�����ݼӾ۷�Ӧԭ������д��E��F��Ӧ�Ļ�ѧ����ʽΪ�� ��
��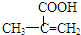 �ķ�����ɡ�ͬ���칹�����дԭ������Ҫ��д�������������л���Ľṹ��ʽ��
�ķ�����ɡ�ͬ���칹�����дԭ������Ҫ��д�������������л���Ľṹ��ʽ��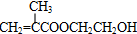 ��B���Ҷ�����Ӧ����C����B�Ľṹ��ʽΪ��
��B���Ҷ�����Ӧ����C����B�Ľṹ��ʽΪ��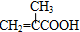 ��Aͨ����������Ӧ������H+��Ӧ����B����A�Ľṹ��ʽΪ��
��Aͨ����������Ӧ������H+��Ӧ����B����A�Ľṹ��ʽΪ��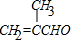 ��
��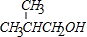 ����F�ܹ�������Ȼ����F�Ľṹ��ʽΪ��
����F�ܹ�������Ȼ����F�Ľṹ��ʽΪ��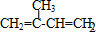 �����ݼӾ۷�Ӧԭ������д��E��F��Ӧ�Ļ�ѧ����ʽΪ��
�����ݼӾ۷�Ӧԭ������д��E��F��Ӧ�Ļ�ѧ����ʽΪ�� ��
��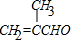 ������A�к��й�����Ϊ��ȩ����̼̼˫����
������A�к��й�����Ϊ��ȩ����̼̼˫����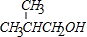 ��Dͨ����ȥ��Ӧ����E��
��Dͨ����ȥ��Ӧ����E��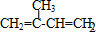 �������ϩͨ���Ӿ۷�Ӧ���������ϩ����Ȼ����
�������ϩͨ���Ӿ۷�Ӧ���������ϩ����Ȼ����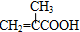 ��B���Ҷ�����Ӧ�Ļ�ѧ����ʽΪ��
��B���Ҷ�����Ӧ�Ļ�ѧ����ʽΪ�� ��
��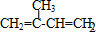 ��Ӧ�Ļ�ѧ����ʽΪ��
��Ӧ�Ļ�ѧ����ʽΪ�� ��
�� ��
�� ��
��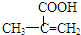 ���ò��������ж���ͬ���칹�壬���������Һ���̼̼˫����ͬ���칹��Ľṹ��ʽ�У�
���ò��������ж���ͬ���칹�壬���������Һ���̼̼˫����ͬ���칹��Ľṹ��ʽ�У�

 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��22.4L�����к�������ӵ���ĿΪNA |
| B��1 mol������O3���к�����ԭ�ӵ���ĿΪ2NA |
| C�����³�ѹ�£�14g�������е�ԭ����ĿΪNA |
| D��0.5 mol/LFe2��SO4��3��Һ�У�SO42-����ĿΪ1.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
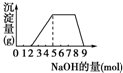 ij��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����������Һ��NaOH�����ʵ����ı仯��ͼ��ʾ��[��֪��Mg2++2OH-�TMg��OH��2����Fe3++3OH-�TFe��OH��3������Mg��OH��2��Fe��OH��3������ˮ��NH4++OH-�TNH3?H2O]�ɴ˿�֪������Һ�п϶����е�������
ij��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����������Һ��NaOH�����ʵ����ı仯��ͼ��ʾ��[��֪��Mg2++2OH-�TMg��OH��2����Fe3++3OH-�TFe��OH��3������Mg��OH��2��Fe��OH��3������ˮ��NH4++OH-�TNH3?H2O]�ɴ˿�֪������Һ�п϶����е��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
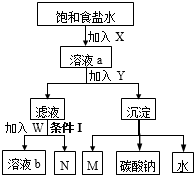
| A��X��M��ͬ������ |
| B��W���Ȼ��ƹ����X |
| C����Һb��N��ѭ������ |
| D���������Ǽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | ��Һ�м��������� |
| ��һ�� | KCl��K2SO4��Na2CO3��NaCl |
| �ڶ��� | KCl��BaCl2��Na2CO3��K2CO3 |
| ������ | Na2CO3��KCl��K2SO4��NaCl |
2- 4 |
2- 3 |
| ʵ�鲽�� | ʵ����� | ʵ��Ŀ�� | ��Ӧ�����ӷ���ʽ | ||
| ��һ�� | ����Һ�еμӹ�����HNO3��Һ | ���� | |||
| �ڶ��� | �����μӹ����� | ����SO
| |||
| ������ | ���ˡ�������Һ�еμ� | ���� | Ag++Cl-=AgCl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com