
��16�֣�ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�ã�ͼ3����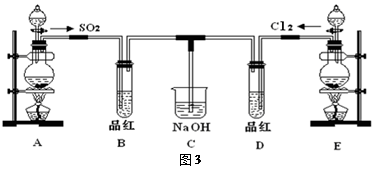
��1���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B��________________________________��D��____________________________��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ
B��________________________________��D��____________________________��
��2����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1:1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ��������������������������������ԭ���û�ѧ����ʽ��ʾ��______________________________ ��
��3��װ��E����MnO2��Ũ���ᷴӦ�Ƶ�Cl2������Ӧ���ɵ�Cl2���Ϊ2.24L����״��������������HClΪ mol��
��4��ʵ���������ͬѧ��Ϊװ��C�п��ܺ���SO32����SO42����Cl����OH���������ӣ�����д��������SO42����SO32����ʵ�鱨�档
��ѡ�Լ���2 mol��L��1 HCl��1 mol��L��1 H2SO4��l mol��L��1 BaCl2��l mol��L��1MgCl2
1 mol��L��1 HNO3��0.1 mol��L��1 AgNO3�����Ʊ�����ˮ��
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | ȡ��������Һ���Թ��У����� ������ | ��֤������Һ�к�SO32���� |
| ����� | �ڲ���ٵ���Һ�е������� | �� ֤������Һ�к�SO42���� |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
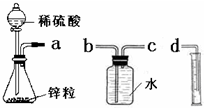 ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮| ������Լ� | H2���������ͬ�����£� | ��Ӧʱ�� | ��Ӧ���� |
| 1mol/L���� | 10mL | t1 | v1 |
| 4mol/L���� | 10mL | t2 | v2 |
| ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ����Ũ�ȣ�mol/L | 4.0 | 3.8 | 3.5 | 3.1 | 2.6 | 2.2 | 1.9 | 1.8 | �� |
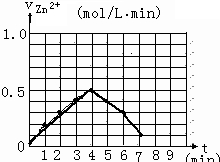
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com