
| O | - 3 |


 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2��AlCl3��NaOH��Һ��Ӧ����Al3+��OH-�����ʵ���֮��Ϊ________ʱ������࣬________ʱ����ȫ���ܽ⣬________ʱ����Ϊ���ʱ��һ�롣
��3���ִ���ѧʵ��֤������Һ�в�����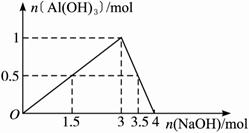 ��ӦΪ��Al��OH��-4�ݣ�
��ӦΪ��Al��OH��-4�ݣ� �����þɵ���дϰ�ߡ���д��Al2��SO4��3��Һ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��������ƫ�����α�ʾ����______________________��
�����þɵ���дϰ�ߡ���д��Al2��SO4��3��Һ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��������ƫ�����α�ʾ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)������з���ʽ����д(���м����л�����ֻд��Ҫ����)��
(1)��ϩ��HBr��Ӧ��_________________________________________��
(2)1��3������ϩ��������Br2�����ʵ�����Ӧ��______________________________��
(3)2���嶡�鷢����ȥ��Ӧ��___________________________________��
(4)�ױ���Cl2���������µ����ʵ�����Ӧ��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ��ӱ�ʡ��ɽһ�и߶�3���¿���ѧ�Ծ� ���ͣ������
(8��)������з���ʽ����д(���м����л�����ֻд��Ҫ����)��
(1)��ϩ��HBr��Ӧ��
____________________________________________________��
(2)1��3������ϩ��������Br2�����ʵ�����Ӧ��
_____________________________________________________��
(3)2���嶡�鷢����ȥ��Ӧ��
_____________________________________________________��
(4)�ױ���Cl2���������µ����ʵ�����Ӧ��
___________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com