
| ���� | �е�/�� |
| �����״� | 380 |
| ���� | 34.6 |
| �屽 | 156.2 |
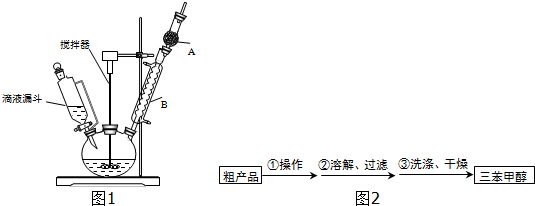
���� ��1��ͼ�в�������B�������������ܣ����ڸ����Լ�����ˮ�⣬B�������Ƿ�ֹ�����е�ˮ��������װ�ã�
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������ƽ��ѹǿ��
��3�������״��ֲ�Ʒ�к������ѡ��屽���������������л�������״��ķе���ߣ�������������ķ�����ȥ�л����ʣ���ʽ�廯þ����ˮ���������л��ܼ�������ϴ��Һѡ��ˮ�����ϴ���Ƿ�ɾ���ȡ�������һ��ϴ��Һ���Թ��У�����������Һ�����Ƿ��������ӣ�
��4����2-OH��H2�ɼ���������״������ʵ������ٸ���m=nM���������״������������������Ʒ�������״�������������
��� �⣺��1��ͼ�в�������B�������������ܣ����ڸ����Լ�����ˮ�⣬B�������Ƿ�ֹ�����е�ˮ��������װ�ã������ʽ�Լ�ˮ�⣬
�ʴ�Ϊ�������ܣ���ֹ�����е�ˮ��������װ�ã���������Լ�ˮ�⣻
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������ƽ��ѹǿ��ʹ©����Һ��˳�����£�
�ʴ�Ϊ��ƽ��ѹǿ��ʹ©����Һ��˳�����£�
��3�������״��ֲ�Ʒ�к������ѡ��屽���������������л�������״��ķе���ߣ�����������������ķ�����ȥ�л����ʣ�
���ڼ�ʽ�廯þ����ˮ���������л��ܼ�������ϴ��Һѡ��ˮ����ѡa��
���ϴ���Ƿ�ɾ���һ�㲽���ǣ�ȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ�����ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
�ʴ�Ϊ����������a��ȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ�����ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
��4����2-OH��H2����֪�����״������ʵ�����$\frac{0.1008L}{22.4L/mol}$��2=0.009mol�����Բ�Ʒ�������״���������0.009mol��260g/mol=2.34g�����Ʒ�������״�����������=$\frac{2.34g}{2.60g}$��100%=90%��
�ʴ�Ϊ��90%��
���� ���⿼���л���ϳ�ʵ�顢���ʵķ�����ϴ�ӵȻ�����������ʵ��װ�õķ������۵ȣ��ϺõĿ���ѧ�������ݵ�Ӧ�á��Ķ���ȡ��Ϣ�������Լ�֪ʶǨ��Ӧ�ã��Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
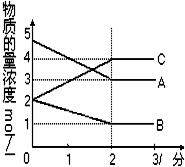 ���ܱ������У�ij��Ӧ�ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ
���ܱ������У�ij��Ӧ�ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ 2C��
2C���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��C�Ļ�ѧʽΪMg��OH��2
��C�Ļ�ѧʽΪMg��OH��2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢ� | B�� | �ڢۢܢݢ� | C�� | �ݢڢ٢ۢ� | D�� | �ܢ٢ڢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ܽ��ԣ������������ܼ��� | �е㣨�棩 | �ܶȣ�g/mL�� | |
| �Ҵ� | ��ˮ���ܣ��������л��ܼ� | 78.5 | 0.8 |
| ������ | ������ˮ���������л��ܼ� | 38.4 | 1.4 |
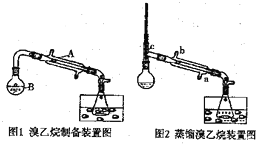
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
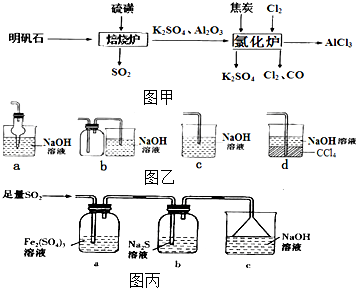 ��ˮ�Ȼ�����һ����Ҫ�Ļ���ԭ�ϣ���������ʯ[K2SO4•A12��SO4��3•2A12O3•6H2O]�Ʊ���ˮ�Ȼ�����������ͼ�ף�
��ˮ�Ȼ�����һ����Ҫ�Ļ���ԭ�ϣ���������ʯ[K2SO4•A12��SO4��3•2A12O3•6H2O]�Ʊ���ˮ�Ȼ�����������ͼ�ף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | ������ | ��״ | �ܶ�g/cm3 | �۵�� | �е�� | �ܽ�ȣ���/100ml�ܼ� | ||
| ˮ | �� | �� | ||||||
| ����ȩ | 106 | ��ɫҺ�� | 1.06 | -26 | 178-179 | 0.3 | ���� | ���� |
| ������ | 102 | ��ɫҺ�� | 1.082 | -73 | 138-140 | 12 | �� | ���� |
| ����� | 148 | ��ɫ�ᾧ | 1.248 | 133-134 | 300 | 0.04 | 24 | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��Ӧʱ��/min | n��CO��/mol | n��H2O��/mol |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
| A�� | ��Ӧ��t1 min�ڵ�ƽ������Ϊv��H2��=$\frac{0.40}{{t}_{1}}$ mol•L-1•min-1 | |
| B�� | ���������������䣬��ʼʱ�������г���0.60 mol CO��1.20 mol H2O���ﵽƽ��ʱn��CO2��=0.40 mol | |
| C�� | ���������������䣬��ƽ����ϵ����ͨ��0.20 mol H2O����ԭƽ����ȣ��ﵽ��ƽ��ʱCOת���ʲ��䣬H2O������������� | |
| D�� | �¶�������800�棬������Ӧƽ�ⳣ��Ϊ0.64��������ӦΪ���ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com