
���ô��ܽ��ʴ���̫���ܵ�ԭ���ǣ�������̫����������ij�ֹ�̬���ۻ�(ʵ�����������������Ľᾧˮ)�������������������ͷų���Ӧ���������̣�����ʹ���µ��Ե��ڡ�
��֪�����ε��۵㼰���ۻ�ʱ�����ı�ֵ���2-1-2��ʾ��
��2-1-2
|
�� |
�۵�(��) |
���ۻ�ʱ����(kJ/mol) |
|
|
29.0 |
37.3 |
|
|
32.4 |
77.0 |
|
|
36.1 |
100.1 |
|
|
48.5 |
49.7 |
���е���������õ�����
[����]
A��
B��
C��
D��
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
| �� | �۵�/�� | �ۻ�����/KJ?mol-1 | �ο��۸�/Ԫ?kg-1 |
| CaCL2?6H2O | 29��0 | 37��3 | 780��850 |
| Na2SO4?10H2O | 32��4 | 77��0 | 800��900 |
| Na2HPO4?12H2O | 36��1 | 100��1 | 1600��2000 |
| Na2S2O3?5H2O | 48��5 | 49��7 | 1400��1800 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� | �۵�/�� | �ۻ�����/kJ��mol-1 | �ο��۸�/Ԫ��kg-1 |
CaCl2��6H2O | 29.0 | 37.3 | 780��850 |
Na2SO4��10H2O | 32.4 | 77.0 | 800��900 |
Na2HPO4��12H2O | 36.1 | 100.1 | 1 600��2 000 |
Na2SiO3��5H2O | 48.5 | 49.7 | 1 400��1 800 |
������������Ϊ���ܽ��ʵ���( )
A.CaCl2��6H2O B.Na2SO4��10H2O
C.Na2HPO4��12H2O D.Na2SiO3��5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣���Ҫ��ش��������⣺
��1����֪��200�棬101kPaʱ��H2���������������1mol HI�ų�7.45kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��2��̫���ܵĿ�����������21����һ����Ҫ���⡣���ô��ܽ��ʴ���̫���ܵ�ԭ���ǣ�������̫��������ʹij�����ۻ��������������������ι̻��ͷų���Ӧ��������֪�������ݣ�
| ��� | �� | �۵�/�� | �ۻ���/ | �ο��۸�/ |
| �� | CaCl2��6H2O | 29.0 | 37.3 | 780��850 |
| �� | Na2SO4��10H2O | 32.4 | 77.0 | 800��900 |
| �� | Na2HPO4��12H2O | 36.1 | 100.1 | 1800��2400 |
| �� | Na2SiO3��5H2O | 52.5 | 49.7 | 1400��1800 |
������������Ϊ���ܽ��ʵ��� ����ѡ��������ţ�
��3�����Ӿ��徧���ܵĶ�������̬�����γ�1mol���Ӿ����ͷŵ�������ͨ��ȡ��ֵ������֪��
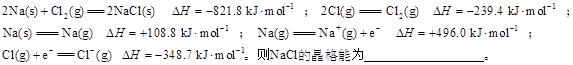
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����ʡ�Ƹ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��6�֣���Ҫ��ش��������⣺
��1����֪��200�棬101kPaʱ��H2���������������1mol HI�ų�7.45kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��2��̫���ܵĿ�����������21����һ����Ҫ���⡣���ô��ܽ��ʴ���̫���ܵ�ԭ���ǣ�������̫��������ʹij�����ۻ��������������������ι̻��ͷų���Ӧ��������֪�������ݣ�
| ��� | �� | �۵�/�� | �ۻ���/ | �ο��۸�/ |
| �� | CaCl2��6H2O | 29.0 | 37.3 | 780��850 |
| �� | Na2SO4��10H2O | 32.4 | 77.0 | 800��900 |
| �� | Na2HPO4��12H2O | 36.1 | 100.1 | 1800��2400 |
| �� | Na2SiO3��5H2O | 52.5 | 49.7 | 1400��1800 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��6�֣���Ҫ��ش��������⣺
��1����֪��200�棬101kPaʱ��H2���������������1mol HI�ų�7.45kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��2��̫���ܵĿ�����������21����һ����Ҫ���⡣���ô��ܽ��ʴ���̫���ܵ�ԭ���ǣ�������̫��������ʹij�����ۻ��������������������ι̻��ͷų���Ӧ��������֪�������ݣ�
|
��� |
�� |
�۵�/�� |
�ۻ���/ |
�ο��۸�/ |
|
�� |
CaCl2��6H2O |
29.0 |
37.3 |
780��850 |
|
�� |
Na2SO4��10H2O |
32.4 |
77.0 |
800��900 |
|
�� |
Na2HPO4��12H2O |
36.1 |
100.1 |
1800��2400 |
|
�� |
Na2SiO3��5H2O |
52.5 |
49.7 |
1400��1800 |
������������Ϊ���ܽ��ʵ��� ����ѡ��������ţ�
��3�����Ӿ��徧���ܵĶ�������̬�����γ�1mol���Ӿ����ͷŵ�������ͨ��ȡ��ֵ������֪��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com