
ij��ѧ̽��ѧϰС���������ͼװ����ȡ���ᣨ�гֺͼ�������������ȥ����ʵ���пɹ�ʹ�õ�ҩƷ�У�Na2CO3��NaHCO3��NH4HCO3��Na2O2��NaOH��Һ��ˮ��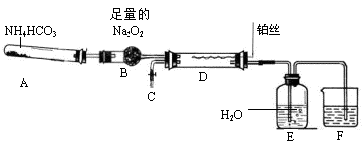
��ش��������⣺
��1��װ��A�з����Ļ�ѧ��Ӧ����ʽ�� ��
��2����ȥװ��D�еļ���װ�ú�˿��Ȼ���ֺ��ȣ�������ΪD�з����Ļ�ѧ��Ӧ��һ�� ������ȡ������ȡ�����Ӧ��
��3��װ��F��ʢ�ŵ��� ��Һ���������� ��
��4��ʵ������У�ҪʹNH4HCO3���ת��ΪHNO3����Ҫ��װ��D��ͨ���������������ͬѧ������C������һ����ȡ������װ�ã���ͬѧ��Ϊ��ֱ����A���ټ��������ṩҩƷ�е�һ�����ʣ�����ҩƷ�Ļ�ѧʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ����������Һ |
| ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2012��2013ѧ���һ��ѧ����ĩ����ģ������(1)����ѧ ���ͣ�058
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ�Ͻ�(�費������Ԫ��)��þ����������������������ֲ�ͬʵ�鷽������̽������д���пհף�
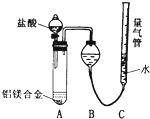
[̽��һ]ʵ�鷽������þ�Ͻ�![]() �ⶨʣ���������
�ⶨʣ���������
�������ۣ�(1)��ȡһ����������þ�Ͻ��ĩ��Ʒ�����������NaOH��Һ����ַ�Ӧ��ʵ���з�����Ӧ�Ļ�ѧ����ʽ��________��
(2)���ˡ�ϴ�ӡ��������ʣ����壮��δϴ�ӹ��壬���þ������������________(�ƫ�ߡ���ƫ�͡�)��
[̽����]ʵ�鷽������þ�Ͻ�![]() �ⶨ������������
�ⶨ������������
ʵ��װ�ã�

�������ۣ�(1)ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ��װ�м�ʯ�ҵĸ���װ�ã��������ǣ�________(���Ҫ������Ҫ��)��
(2)Ϊȷ�ⶨ��������������ʵ����Ӧע���������(ֻҪ��д������һ��)��________
[̽����]
ʵ�鷽��������xg��þ�Ͻ��ĩ����������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������գ�

�������ۣ�(1)������Mg��������������ʵ���л���ⶨ��������________��
(2)���ÿ�������O2����ʵ�飬�Բⶨ����Ƿ���Ӱ�죿________(��ǡ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ�Ͻ��費������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհף�
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ�Ͻ��費������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհף� �ⶨʣ���������
�ⶨʣ��������� �ⶨ������������
�ⶨ�������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ̽��ѧϰС������������װ�òⶨij��ɫ��������������Ļ�ѧʽ������������������ʣ�����Ҫ��ѧ��ӦΪ��
��H2C2O4���Ҷ��ᣩCO2��+CO��+H2O ��Fe![]() O
O![]() +
+![]() CO=
CO=![]() Fe+
Fe+![]() CO2
CO2
����Ҫ�ⶨ�������ǿ��������ͷ�Ӧ�������ɵ�CO2������[��m��Fe![]() O
O![]() ����m��CO2����ʾ]��������������ͬҩƷ����ʾ��ͼ���£���Ҫʱ�е��������ظ�ʹ�ã�
����m��CO2����ʾ]��������������ͬҩƷ����ʾ��ͼ���£���Ҫʱ�е��������ظ�ʹ�ã�
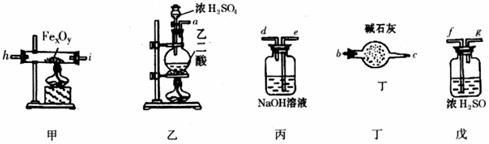
��1������ϴ������������װ�ñ����������ڵķ�Ӧ���еö���ȫ��������������ҵ���������������װ�������ýӿ���ĸa��b������ʾ����
�� �� �� �� �� �� �� �� c �� f
��2��ʵ������һ������ǰ���õ����Σ�ǰ��������Ŀ���� �������������Ŀ���� ��
��3��Ϊ�˱���������������������ĩ�˵�����CO��д�����巽����
��4��������m��Fe![]() O
O![]() ��=15��2g��m��CO2��=11��0g����x��yΪ
��=15��2g��m��CO2��=11��0g����x��yΪ
A��4��5 B��1��1 C��2��3 D��3��4
�ڸ�ʵ���У���ӦǰFe![]() O
O![]() Ϊ��ɫ����Ӧ�����ɵ�FeҲΪ��ɫ������ȷ��Fe
Ϊ��ɫ����Ӧ�����ɵ�FeҲΪ��ɫ������ȷ��Fe![]() O
O![]() �Ƿ���ȫ����ԭ��������Fe
�Ƿ���ȫ����ԭ��������Fe![]() O
O![]() ʣ�࣬�������x��yֵ��ʵ��ֵ ����ƫ�ͣ�ƫ��Ӱ�죩��ȡʲô��ʩ���Ա�������ԭ�������żȻ��
ʣ�࣬�������x��yֵ��ʵ��ֵ ����ƫ�ͣ�ƫ��Ӱ�죩��ȡʲô��ʩ���Ա�������ԭ�������żȻ��
��5�����������ṩ��������ҩƷ����Ҫʱ������ѡ��Ҳ�����µ�˳�������װ����ͬ���ⶨ�������ʵ���������ɼ����x��y�ı�ֵ��д��Ҫ�ⶨ���������ݿ��ܵ���ϣ�
�� �� ��
��������������ϣ��ɲ���������������������ϣ����������ӣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com