
| O | 2- 4 |
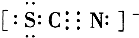 ��
��| O | 2- 4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
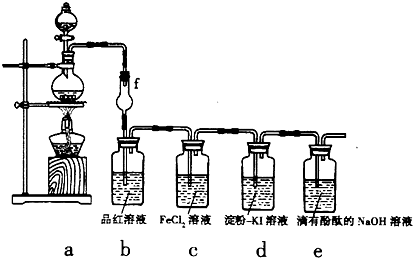
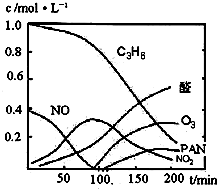 ����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮
����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮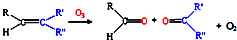 ��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��
��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��
| ||
| ||
| K22 |
| K14 |
| K22 |
| K14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ӡ�ʵ���У�3I-+S2O82-=I3-+2SO42-�ķ�Ӧ���ʿ�����I3-�����ĵ�����Һ����ɫ��ʱ��t��������tԽС����Ӧ����Խ��ij̽����ѧϰС����20�����ʵ�飬�õ������������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
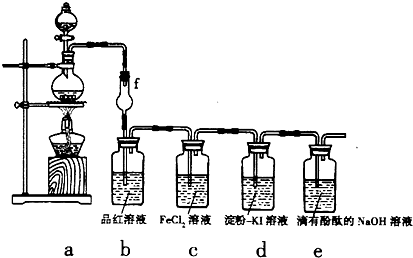
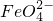 ����
����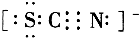 ��
��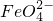 ����д���÷�Ӧ�����ӷ���ʽ��______��
����д���÷�Ӧ�����ӷ���ʽ��______���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com