
����Ŀ�������£���0.10 molL-1NH3H2O����Һ�ζ�20 mL0.10 molL-1������δ֪Ũ��CH3COOH�Ļ����Һ�������Һ����Ե��������仯������ͼ��ʾ����֪Kb(NH3��H2O) =Ka(CH3COOH)�����������������
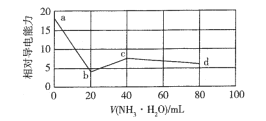
A.H+�ĵ����������ڵ�Ũ�ȵ�NH4+
B.a������Һ��c( CH3COO- ) +c( CH3COOH) =0.10 molL-1
C.b������Һ�У�c( NH4+ ) +c( NH3H2O) >c( CH3COOH)
D.c������Һ�У�c(NH4+) >c(Cl-) >c(CH3COO-) >c(OH-)>c(H+)
���𰸡�D
��������
����ͼ����Ϣ����20mL 0.10 molL1 NH3H2Oʱ����������ͣ����ʱ20 mL0.10 molL1����ǡ�÷�Ӧ�꣬�ټ���20mL 0.10 molL1 NH3H2Oʱ�����������ӵ�������˵����40mLʱCH3COOH�����꣬��˵��CH3COOH��Ũ��Ϊ0.10 molL1���ټ���NH3H2Oʱ���������½����������Һ�����������Ũ�ȼ�С��
A. a��������ʹ���Ļ����Һ��Ũ����ȣ�b��������շ�Ӧ��ȫ��b��������NH4Cl��CH3COOH�Ļ����Һ�����Դ���������Һ����Ӻ͵ı仯����Һ�����Ϊ40mL��b��NH4Cl��a������Ũ�ȵ�һ�룬���H+�ĵ����������ڵ�Ũ�ȵ�NH4+����b�㵼������Ӧ����a�㵼��������һ�룬��b�㵼��������a�㵼������һ�㻹С��˵��H+�ĵ����������ڵ�Ũ�ȵ�NH4+����A��ȷ��
B. ���ݷ���ԭ20mL��Һ��CH3COOH��Ũ��Ϊ0.10 molL1�����a������Һ��c(CH3COO��) +c( CH3COOH) =0.10 molL1����B��ȷ��
C. b������ΪNH4Cl��CH3COOH�Ļ����Һ������Ũ����ȣ����������غ㣬c(NH4+) +c(NH3H2O) = c(CH3COOH) + c(CH3COO��)�����c(NH4+) +c(NH3H2O)�� c(CH3COOH)����C��ȷ��
D. c������Һ����ΪNH4Cl��CH3COONH4��NH4+��CH3COO��ˮ�⣬����Kb(NH3��H2O) =Ka(CH3COOH)�����CH3COONH4�����ԣ�NH4Cl�����ԣ���˻����Һ�����ԣ���D����
������������ΪD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijŨ�ȵİ�ˮ�д�������ƽ�⣺NH3H2O![]() NH4++OH-����������NH4+��Ũ�ȣ���������OH-��Ũ�ȣ�Ӧ��ȡ�Ĵ�ʩ�ǣ�������
NH4++OH-����������NH4+��Ũ�ȣ���������OH-��Ũ�ȣ�Ӧ��ȡ�Ĵ�ʩ�ǣ�������
���ʵ������¶� �ڼ���NH4Cl ���� ��ͨ��NH3 �ܼ�����������
A. �٢�B. �ڢ�C. �ۢ�D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Դ�ǵ����о���һ���ȵ����⡣������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ����(ѹ��2.0��10.0Mpa���¶�230��280��)�������з�Ӧ��
��CO(g)+2H2(g)CH3OH(g) ��H1=-99kJ��mol1
��2CH3OH(g)CH3OCH3(g)+H2O(g) ��H2=-23.5kJ��mol1
��CO(g)+H2O(g)CO2(g)+H2(g) ��H3=-41.2kJ��mol1
(1)����Ӧ���е��ܷ�Ӧ3CO(g)+3H2(g)CH3OCH3(g)+CO2(g)��������H=_______����Ӧ����ú����������֪�÷�Ӧ��ƽ�ⳣ������ʽΪK=![]() ��ÿ����1mol H2��Ҫ����131.3kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________��
��ÿ����1mol H2��Ҫ����131.3kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________��
(2)�ڸ������£�����Ӧ�ٵ���ʼŨ�ȷֱ�Ϊ��c(CO)=0.6mol��L1��c(H2)=1.4mol��L1��8min��ﵽƽ�⣬CO��ת����Ϊ50%����8min��H2��ƽ����Ӧ����Ϊ__________��
(3)��t��ʱ����Ӧ�ڵ�ƽ�ⳣ��Ϊ400�����¶��£���1L���ܱ������м���һ���ļ״�����Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
c(mol��L1) | 0.46 | 1.0 | 1.0 |
��ʱ��v��___v��(���������������)��ƽ��ʱc(CH3OCH3)�����ʵ���Ũ����___��
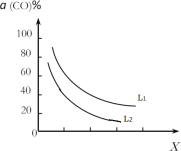
(4)��(1)С���д���Ӧ�ҵ��ܷ�Ӧ3CO(g)+3H2(g)CH3OCH3(g)+CO2(g)��CO��ƽ��ת����a(CO)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��ͼ��X����___(��¶ȡ���ѹǿ��)����L1___L2(���������������)��
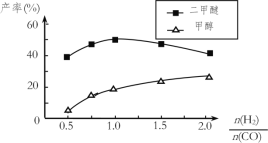
(5)�ڴ�����������ͬʱ����������Ӧ������������ʼͶ�ϱ�![]() �ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��_____��
�ĸı䣬�����Ѻͼ״��IJ���(�����е�̼ԭ��ռ��ʼCO��̼ԭ�ӵİٷ���)������ͼ�ı仯���ơ��Խ���Ͷ�ϱȴ���1.0֮������Ѳ��ʺͼ״����ʱ仯��ԭ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ʵ�� | �Լ��� | �Լ��� | �Լ��� | ʵ����� |
|
A | Ũ���� | ͭƬ | ������KI��Һ | �����ԣ�NO2��I2 | |
B | ϡ���� | FeS | ��AgNO3��AgCl��Һ | Ksp(AgCl)��Ksp(Ag2S) | |
C | Ũ��ˮ | CaO | ��ɫʯ����Һ | ��ˮ�ʼ��� | |
D | ϡ���� | ʯ��ʯ | BaCl2��Һ | ��������BaCO3���� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����յ�ҹ������������ͯ�ֳ�ӫ�����Ϸ��ħ������ԭ��������H2O2�������������CPPO�����������������������ݸ�ӫ�����ʺ�ӫ�⣮��������ṹ��ʽ��ͼ��ʾ�������йز��������˵������ȷ���ǣ�������

A. ���������H2��ȫ��Ӧ����Ҫ6mol H2
B. ��������ķ���ʽΪC26H24O8Cl6
C. ��������ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ
D. 1mol�������������4mol NaOH��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ������ڵ�һ����,�����Ԫ�آ�~���ڱ��е�λ��,�ش��������⡣
(1)Ԫ�آ������ڱ��е�λ����______________��
(2)Ԫ�آݺ͢��γɵĻ�����ĵ���ʽΪ__________________��
(3)Ԫ�آܡ��ݡ����γɵļ����ӵİ뾶����__________________(��������������С������������)��
(4)����Ԫ�آڡ��ۡ�����ۺ������������ǿ������˳����_____________(�ѧʽ)��
(5)Ԫ�آ��γɵĵ��ʿ���ݵ�����������Ӧ��ˮ�������Ӧ,�䷴Ӧ�����ӷ���ʽΪ___________
(6)��һ��������,����ۿ��γ�һ�ֻ�����X,����Է���������O2��ͬ,��X���ڴ�����ȼ��,���ò���Ի���������Ⱦ,��Xȼ�յĻ�ѧ����ʽΪ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�����ڱ���һ������ͼ��ʾ,W��X��Y��Z��Ϊ����������Ԫ��,X��Z���������֮����W����ȡ�����˵���������
![]()
A.ԭ�Ӱ뾶:X>Y>Z>W
B.X2W2�к������Ӽ����ۼ�
C.Y������������Ӧ��ˮ����������ˮ
D.����X��Z�ĵ�����ɵĻ���������ˮ��,Z�ĵ��ʲ�������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���ݻ��̶���5 L��Ӧ���У���һ�����һ������ܷ���壬���������¿��淴Ӧ��2SO2(g)+O2(g)![]() 2SO3(g)��ͨ�������¶�ʹ��ﵽ��ͬ��ƽ��״̬���Ҳ���뵪���������¶ȱ�����ȡ�����м���SO2��O2��SO3�����ʵ����ֱ�Ϊx mol��3.25 mol��1 mol���Ҳ��м���9 mol�ĵ�������෴Ӧ�ﵽƽ��ʱ������ǡ�ô��ڷ�Ӧ��λ��2����������˵���������
2SO3(g)��ͨ�������¶�ʹ��ﵽ��ͬ��ƽ��״̬���Ҳ���뵪���������¶ȱ�����ȡ�����м���SO2��O2��SO3�����ʵ����ֱ�Ϊx mol��3.25 mol��1 mol���Ҳ��м���9 mol�ĵ�������෴Ӧ�ﵽƽ��ʱ������ǡ�ô��ڷ�Ӧ��λ��2����������˵���������
![]()
A.����ʼ��Ӧʱv��>v������5>x>1.75
B.����ʼ��Ӧʱx��1.75������ʼʱv����v��
C.����ʼ��Ӧʱx��2����ﵽƽ��ʱ���������SO2��ռ���������Ϊ25%
D.����ʼ��Ӧʱx��1.65����ﵽƽ��ʱSO3��ת����Ϊ10%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С����Ƴ����ù�ҵ����(ϡ����)����ȡij����������ͭп��ķ�����ʵ�ַ����ۺ����ã�������ͼ��ʾ��

��֪�������ӿ�ʼ��������ȫ����ʱ��pH�����ʾ��

��ش��������⣺
(1)�ڡ�����������У�Ϊ��߽������ʣ���ͨ����������衱�⣬���ɲ�ȡ�Ĵ�ʩ��(��дһ�㼴��)__________________________________________________________��
(2)����A���ʹ�����������е�____________����ѡ����ţ���
A��KMnO4����B��H2O2����C��HNO3
(3)���������м��백ˮ��Ŀ���ǵ�����Һ��pH��pHӦ������__________��Χ֮�䡣
(4)����B��ֱ���������ʣ���B�Ļ�ѧʽ��________��
(5)������õ��������������ô��������Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ�ܴ�����(K2FeO4)��д���÷�Ӧ�����ӷ���ʽ��__________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com