
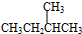 ��
�� G��
G��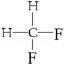 ��
�� 
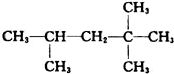 ����������ȼ��Ʒ�ʿ������ܵIJ��������A��ͬ���칹���к���Ч��ԭ���������ٵ�һ�ֽṹ��ʽΪ��CH3C��CH3��2C��CH3��2CH3����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ������ϩ�������п��ܵĽṹ��ʽΪ
����������ȼ��Ʒ�ʿ������ܵIJ��������A��ͬ���칹���к���Ч��ԭ���������ٵ�һ�ֽṹ��ʽΪ��CH3C��CH3��2C��CH3��2CH3����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ������ϩ�������п��ܵĽṹ��ʽΪ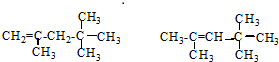 ��
��
���� ��1����������ͬ��������������������ͬ��ԭ�ӻ���ͬλ�أ�ͬϵ��ָ�ṹ���ơ�ͨʽ��ͬ����������1���������ɸ�CH2ԭ���ŵĻ������������Ŀ��������ȣ�������ͬ����ʽ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻��ɺͽṹ����ͬ������Ϊͬһ���ʣ�ͬһ������ɡ��ṹ�����ʶ���ͬ���ṹʽ����״�����ʵľۼ�״̬���ܲ�ͬ��
��2���ٸ��¶���ˮΪ���壬���ݷ�Ӧ����ʽ2CO+O2=2CO2��C2H4+3O2=2CO2+2H2O��g������������CO��C2H4����ͬ��������ȫȼ�գ�����CO2������ȡ�����O2������ȣ�
�����CO��C2H4�������Ȼ��������������ȫȼ���������������ɶ�����̼�������������������һ����̼����ϩ�������
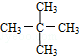
��3����A��ͬ���칹���к���Ч��ԭ���������ٵ�һ�ֽṹ��ʽΪ2��2��3��3-�ļ����飻���̼ԭ���γ�4�����ۼ��ж�ϩ����
�ڽ���Ϊ̼ԭ�ӣ���Hԭ�ӱ���̼���ļ۽ṹ���ݴ��ж�C��Hԭ����Ŀ����д����ʽ�����ݵ�Ч���ж�һ�ȴ�����Ŀ��
��4����������������Ӧ�����ɵ�����ˮ��
��� �⣺��1��A�����ʯ��ʯī��Ϊ̼Ԫ���γɵIJ�ͬ�ֵ��ʣ���Ϊͬ�������壻B����������ά�ؾ����ڸ߷��ӻ�������������Ϲ�ϵ��C������������ͬ����������ͬ������ͬλ�أ�D������������ṹ���ƣ�������������4��CH2ԭ���ţ�����ͬϵ� E������������Ƿ���ʽ��ͬ���ṹ��ͬ������ͬ���칹�壻
F��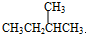 ��
��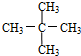 ����ʽ��ͬ���ṹ��ͬ������ͬ���칹�壻 G��
����ʽ��ͬ���ṹ��ͬ������ͬ���칹�壻 G��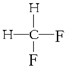 ��
��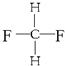 ����ͬ�����ʣ�
����ͬ�����ʣ�
�ʴ�Ϊ��C�� D��EF��G��
��2����120�棬101kpa�£����ɵ�ˮΪ���壬���ݷ�Ӧ����ʽ2CO+O2=2CO2��C2H4+3O2=2CO2+2H2O��g����֪���������CO��C2H4����ͬ��������ȫȼ�գ�����CO2�������ȣ��������Ϊ1��2�����������������Ϊ��$\frac{1}{2}$��3=1��6��
�ʴ�Ϊ��1��2��1��6��
����9mL������к���xmLCO��ymL��ϩ�����x+y=9��
���ݷ�Ӧ2CO+O2=2CO2��C2H4+3O2=2CO2+2H2O��g����֪���������������Ϊ��0.5x+3y��
���ɶ�����̼�����Ϊ��x+2y��
��û��������ȫȼ��������O2�����������CO2�������ȣ���0.5x+3y=x+2y��
���ݢ٢ڽ�ã�$\left\{\begin{array}{l}{x=6}\\{y=3}\end{array}\right.$��
������������к���6mLCO��3mL��ϩ��
�ʴ�Ϊ��6mL��3mL��
��3����A��ͬ���칹���к���Ч��ԭ���������ٵ�һ�ֽṹ��ʽΪ2��2��3��3-�ļ����飬ΪCH3C��CH3��2C��CH3��2CH3����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ������ϩ���Ľṹ��ʽ����Ϊ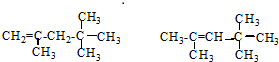 ��
��
�ʴ�Ϊ��CH3C��CH3��2C��CH3��2CH3��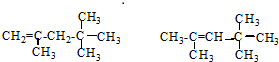 ��
��
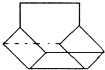
�ڽ���Ϊ̼ԭ�ӣ���Hԭ�ӱ���̼���ļ۽ṹ��������Ľṹ��֪�������к���10��Cԭ�ӡ�12��Hԭ�ӣ�����ʽΪC10H12��
���л���������ڸ߶ȶԳƽṹ������ͼ��ʾ��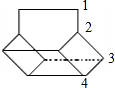 ��4��Hԭ�ӣ�����һ�ȴ�����4�֣�
��4��Hԭ�ӣ�����һ�ȴ�����4�֣�
�ʴ�Ϊ��C10H12�� 4��
��4�������缫��ӦʽΪN2H4+4OH--4e-�T4H2O+N2�����ʴ�Ϊ��N2H4+4OH��-4e��=4H2O+N2����
���� ���⿼���Ϊ�ۺϣ��漰�л���Ľṹ�����ʡ��л����������ͬ���칹���Լ��绯ѧ֪ʶ��Ϊ�߿��������ͣ�������ѧ����˫���Ŀ��飬ע������л���Ľṹ�����ʵ��ж��Լ��缫����ʽ����д���ѶȲ���


 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | m1=m2 | B�� | m1��m2 | C�� | m1��m2 | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
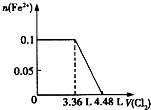 ��1�����е����ʵ�����SO32-��Fe2+��Br-��I-����Һ�У�ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ�����ϵ��ͼ��ʾ��
��1�����е����ʵ�����SO32-��Fe2+��Br-��I-����Һ�У�ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ�����ϵ��ͼ��ʾ��| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �١�����Ԫ�����ڱ���ǰ�����ڵ�8��Ԫ�أ������λ����ͼ��ʾ��
�١�����Ԫ�����ڱ���ǰ�����ڵ�8��Ԫ�أ������λ����ͼ��ʾ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ��FeCl3��Һ��Fe3+��Cl-�ĸ���֮�ȵ���1��3 | |
| B�� | ����Na2CO3��Һ�ɳ����ۣ�CO32-+2H2O?H2CO3+2OH- | |
| C�� | ����ʱ��0.1mol•L-1��������ˮ�������c��H+����10-7mol/L | |
| D�� | �Ȼ�������ˮ�������룬���뷽��ʽΪ��NaCl$\frac{\underline{\;ͨ��\;}}{\;}$Na++Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� SO2ͨ��ˮ�� | B�� | �ռ�����ˮ | C�� | �� HCl ͨ��ˮ�� | D�� | NaHSO4����ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ 8 �����ӵ�̼ԭ�ӣ�68C | B�� | �ʻ���COS���Ľṹʽ��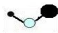 | ||
| C�� | ��ϩ�ı���ģ�ͣ� | D�� | NH4Br �ĵ���ʽ��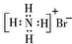 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�߶����¿�һ��ѧ���������棩�� ���ͣ�ѡ����
�������ӷ���ʽ�У���д��ȷ������ˮ�ⷴӦ����
A��SO32-+2H2O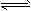 H2SO3+2OH-
H2SO3+2OH-
B��HS-+H3O+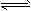 H2S+H2O
H2S+H2O
C��H2S+ OH-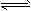 HS-+H2O
HS-+H2O
D��Cu2++ 2H2O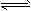 Cu(OH)2+2OH-
Cu(OH)2+2OH-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com