
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
| V��NaOH��/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 |
| ��ҺpH | 2.88 | 4.70 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 |
| ָʾ�� | ��ɫ�ķ�Χ��pH�� |
| ���� | 3.1��4.4 |
| ʯ�� | 5.0��8.0 |
| ��̪ | 8.2��10.0 |
 =15.00ml��c����Ʒ��V����Ʒ��=c��NaOH��V��NaOH��
=15.00ml��c����Ʒ��V����Ʒ��=c��NaOH��V��NaOH�� =4.5g/100ml��
=4.5g/100ml��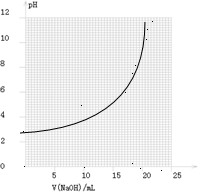 ���ʴ�Ϊ��
���ʴ�Ϊ��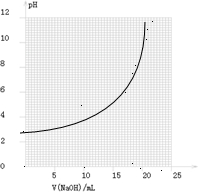



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ����� ʵ������ |
1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
| V��NaOH��/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 |
| ��ҺpH | 2.88 | 4.70 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 |
| ָʾ�� | ��ɫ�ķ�Χ��pH�� |
| ���� | 3.1��4.4 |
| ʯ�� | 5.0��8.0 |
| ��̪ | 8.2��10.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� ʵ������ |
1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ѧ2008�������ѧ�ڶ��ָ�ϰʵ����ѵ�� ���ͣ�058
ijʵ��С����������к͵ζ����ⶨʳ��������(g/100 mL)������������뱾ʵ�鲢�ش�������⣮(�й�ʵ��ҩƷΪ������ʳ�ð״���Ʒ500 mL��0.1000 mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��0.1%ʯ����Һ��)
��ʵ�鲽�裺
(1)�õζ�����ȡ10 mL���۰״���Ʒ������100 mL����ƿ�У�������ˮ(��г�ȥCO2��Ѹ����ȴ))ϡ�����̶��ߣ�ҡ�ȼ��ô���ʳ����Һ��
(2)����ʽ�ζ���ȡ����ʳ����Һ20.00 mL��________�У�
(3)ʢװ��NaOH��Һ�����ú�ȡ���ݣ���¼ΪNaOH����Һ����ij�������
(4)�ζ�������¼NaOH���ն������ظ��ζ�2��3�Σ�
��ʵ���¼�����ݴ���
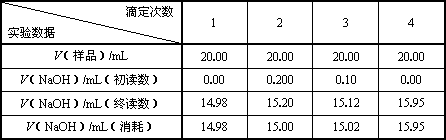
��c(��Ʒ)/moL��L��1��________����Ʒ������g / 100mL��________��
���������ۣ�
(1)��ͬѧ�ڴ������ݹ����м���ã�
V(NaOH)(ƽ������)��1/4(14.98��15.00��15.02��15.95)mL��15.24 mL��
�Է������ļ����Ƿ�����������������˵�����ɣ�
________________________________________________________________��
(2)��ͬѧ��0.1000 mol/L��NaOH��Һ�ζ���һ���۰״���Ʒ��Һʱ���ζ�������ʹ��pH�ƽ���Һ��pH�仯�����¼���±���
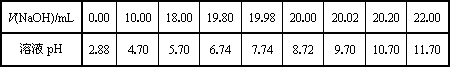
��������pH��V(NaOH)ͼ��
���ɱ���ͼ��֪������������Χ(��0.1%)�ڣ�pHͻ��
(�ζ�ͻԾ)��ΧΪ________��
���Կ�ѡ��________��ָʾ����
��������ָʾ���ı�ɫ��Χ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
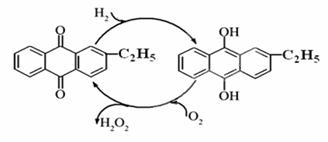
������������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȡ���ش�������⣺
��1��Ŀǰ�����һ��������Ʊ��������⣬��Ҫ��������ͼ���˹��̵��ܷ���ʽΪ ��
��2��ʵ���ó�������������Ϊ0.51%H2O2ˮ��Һ���ܶ�Ϊ1g/mL����pHΪ5
i.д��H2O2����ˮ�ĵ��뷽��ʽ .
ii.�ⶨH2O2ˮ��ҺpH�ķ���Ϊ(����)
A.�����ָʾ���ⶨ B.�ù㷺pH��ֽ�ⶨ
C.�þ���pH��ֽ�ⶨ D.��pH�Ʋⶨ
��3��ijʵ��С�����о�Ũ�ȡ���������Һ����Զ�H2O2�ֽⷴӦ���ʵ�Ӱ�졣�ڳ����°������·������ʵ�顣
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10 mL 2% H2O2��Һ | �� |
| �� | 10 mL 5% H2O2��Һ | �� |
| �� | 10 mL 5% H2O2��Һ | 0.1gMnO2��ĩ |
| �� | 10 mL 5% H2O2��Һ������HCl��Һ | 0.1gMnO2��ĩ |
| �� | 10 mL 5% H2O2��Һ������NaOH��Һ | 0.1gMnO2��ĩ |
i.ʵ��ٺ͢ڵ�Ŀ����_______��ʵ��ʱ����û�й۲쵽������������ó����ۡ�������ʾ��ͨ��������H2O2���ȶ������ֽ⡣Ϊ�˴ﵽʵ��Ŀ�ģ����ԭʵ�鷽���ĸĽ���_________��
ii.ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
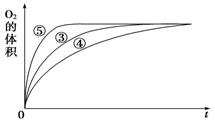
������ͼ�ܹ��ó���ʵ�������____________��
��4��ʵ���ҳ������Ը�����ر���Һ�ⶨ˫��ˮ��Ũ�ȣ���Ӧԭ��Ϊ��
MnO4����H2O2��H�� ��Mn2����H2O�� O2��
i.����ƽ�������ӷ���ʽ
ii.����Һ����ȡ25.00mL����������ƿ�У��ظ��ζ��ĴΣ�ÿ������0.1000 mol��L-1��KMnO4����Һ������±���ʾ��
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| �����mL�� | 17.10 | 18.10 | 18.00 | 17.90 |
���������й��������Ũ��Ϊ mol��L-1��
iii.���ζ�ǰ�����������ݵζ�����ʧ����ⶨ��� ����ƫ�ߡ���ƫ�͡����䡱����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com