
����Ŀ��ij��ѧС��ͨ���������ϣ��������ͼ��ʾ�ķ����Ժ����ϴ���Ϊԭ�����Ʊ�NiSO4��7H2O����֪ij�������ĺ����ϴ�����Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ�����������������ʣ�3.3%��

����������������������ʽ����ʱ��pH�����

��1����Һ���е���������_________���������ʱ�����������____________���ѧʽ����
��2������H2O2ʱ������Ӧ�����ӷ���ʽΪ________________________������bΪ������Һ��pH������ΪpH�ĵ��ط�Χ��___________________��
��3����Ʒ�����л��������̷���FeSO4��7H2O������ԭ�������_______________��д��һ�㼴�ɣ���
��ȡ50�˸ò�Ʒ�������100mL��Һ��ȡ20mL����Һ��0.1mol/L������KMnO4��Һ�ζ�����������KMnO4��Һ10mL��Ni2+��������������ò�Ʒ����Ĵ�����___________��
��4��NiSO4��7H2O�������Ʊ������أ�NiMH����NiMH�е�M��ʾ���������Ͻ𡣸õ���ڳ��������ܷ�Ӧ�Ļ�ѧ����ʽ��Ni(OH)2+M=NiOOH+MH����NiMH��طŵ�����������缫��Ӧʽ________��
���𰸡�OH-��AlO2- H2SO4 H2O2 +2Fe2++2H+ =2Fe3+ + 2H2O 3.2~7.1 H2O2�������㣨����ʱ�䲻���� 86.1% NiOOH+H2O+e-=Ni(OH)2+OH-
��������
�����̿�֪��ij�������ĺ���������Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ�����������������ʣ�3.3%����������˵õ��������������˼����������������������Ϊ�����ӣ�������ҺPHʹ�����Ӻ�������ȫ�������������Ӳ����������˺������ҺPH2-3��ֹ������ˮ�⣬ͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ�NiSO47H2O���壻
��1������������Ҫ����Ni��������Al��31%���ĵ��ʼ���������������������Ժ�ǿ�Ӧ�ܽ�õ���Һ����ƫ�����Σ�����Һ���е���������OH-��AlO2-����������Ʊ�NiSO47H2O����ֹ����������־������Ҫ������������ܽ⣻
��2��������˼�����������Ŀ����������������Ϊ�����ӣ�����H2O2ʱ������Ӧ�����ӷ���ʽΪH2O2 +2Fe2++2H+=2Fe3++2H2O������ͼ���г�����Ҫ����ҺPH�������������������������Ϊ�����Ӻ�����ҺPHʹ������ȫ�������������Ӳ������õ��ϴ�������������Һ��
��3�������̷�����˵���ڼ���������Ᵽ�¹����У���������δ����������ȫ���������ø��������Һ�ζ��������ӷ���������ԭ��Ӧ�����ӷ�ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O�����ݷ�Ӧ����ʽ���������������ʵĺ���������������Ʒ�д��ȣ�
��4���õ���ڳ��������ܷ�Ӧ�Ļ�ѧ����ʽ��Ni(OH)2+M=NiOOH+MH��������NiOOH�õ�������Ni(OH)2��
��1������������Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ�����������������ʣ�3.3%������Һ��Ϊ�������˵õ�����Һ�����������������Ժ�ǿ�Ӧ�ܽ⣬�õ���Һ����ƫ�����Σ�����Һ���е���������OH-��AlO2-����������Ʊ�NiSO47H2O����ֹ����������־������Ҫ������������ܽ⣬
�ʴ�Ϊ��OH-��AlO2-��H2SO4��
��2��������˼�����������Ŀ����������������Ϊ�����ӣ�����H2O2ʱ������Ӧ�����ӷ���ʽΪH2O2 +2Fe2++2H+=2Fe3++2H2O������ͼ���г�����Ҫ����ҺPH�������������������������Ϊ�����Ӻ�����ҺPHʹ������ȫ�������������Ӳ������õ��ϴ�������������Һ��pHӦ��3.2~7.1��
�ʴ�Ϊ��H2O2 +2Fe2++2H+=2Fe3++2H2O��3.2~7.1��
��3����Ʒ��������ʱ����������̷�(FeSO47H2O)��˵���ڼ����������������������������ʱδ����������ȫ������������������������pH���������ӳ�����pH������Ŀ���ǰ�����������ȫ�������ø��������Һ�ζ��������ӷ���������ԭ��Ӧ�����ӷ�ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O����MnO4-~5Fe2+����20mL ��Ʒ��Һ��n(FeSO4)= n(Fe2+)=5n(MnO4-)=5��0.1mol/L��0.01L=0.005mol����100mL����Ʒ��Һ��n(FeSO4)= 0.005mol��![]() =0.025mol����m(FeSO47H2O)= 0.025mol��278g/mol=6.95g��m(FeSO47H2O)+ m(NiSO47H2O)=50g����NiSO47H2O�Ĵ���=
=0.025mol����m(FeSO47H2O)= 0.025mol��278g/mol=6.95g��m(FeSO47H2O)+ m(NiSO47H2O)=50g����NiSO47H2O�Ĵ���=![]() =86.1%��
=86.1%��
�ʴ�Ϊ��H2O2����������(��H2O2ʧЧ)������ʱ�䲻�㵼��Fe2+δ����ȫ������86.1%��
��4��NiMH��طŵ�����У�������NiOOH�õ�������Ni(OH)2���������ĵ缫����ʽΪ��NiOOH+H2O+e-=Ni(OH)2+OH��
�ʴ�Ϊ��NiOOH+H2O+e-=Ni(OH)2+OH-��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���У���ѡ�õ�װ�á�ҩƷ����Ӧԭ������ȷ����
ѡ�� | Ŀ�� | װ�� | ԭ�� |
A | ���������������Ҵ� |
| �����������Ҵ����ܶȲ�ͬ |
B | ʵ�����Ʊ����� |
|
|
C | ֤���ǽ�����N��C��Si |
| ��ۺ��������ԣ����̼��>���� |
D | ��ȥ�����е����� |
| ���������ѵķе㲻ͬ |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij���淴Ӧ��mA(g)+nB(g)![]() xC(g) ����=Q kJmol-1�����ܱ������н��У���ʾ��Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿP��C�ڻ�������еİٷֺ���(C%)�Ĺ�ϵ���ߣ��� ��
xC(g) ����=Q kJmol-1�����ܱ������н��У���ʾ��Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿP��C�ڻ�������еİٷֺ���(C%)�Ĺ�ϵ���ߣ��� ��

A.T1>T2��P1>P2��m+n>x��Q>0B.T1<T2��P1<P2��m+n<x��Q>0
C.T1>T2��P1<P2��m+n<x��Q<0D.T1<T2��P1<P2��m+n>x��Q<0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ʵ���Һ��CH3COOH����HCl����H2SO4����NaHSO4
(1)��������Һ�����ʵ���Ũ����ͬ����c(H+)�Ĵ�С˳��Ϊ______(����ű�ʾ����ͬ)��
(2)��������Һ��c(H+)��ͬ�������ʵ���Ũ�ȵĴ�С˳��Ϊ________��
(3)��6 g CH3COOH����ˮ�Ƴ�1 L��Һ������Һ�����ʵ���Ũ��Ϊ________�����ⶨ��Һ��c(CH3COO-)Ϊ1.4��10��3 mol��L��1�����¶��´���ĵ��볣��Ka=________���¶����ߣ�Ka��______(�������������������������С������ͬ)����������CH3COONa��c(H��) _________��Ka________��ԭ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ����2 L�ܱ������У�������̬����X��Y��Z�����ʵ�����n����ʱ�䣨t���仯��������ͼ��ʾ����ͼ�����ݷ����ɵã�

��1���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________��
��2����Ӧ��ʼ��2 min����Y��ʾ��ƽ����Ӧ����Ϊ_________��X��ת����Ϊ________��
��3����һ���¶��£������������Ϊ������Ӧ�ﵽ��Ӧ�ȵı�־����_______��
A��X��Y��Z��Ũ����� |
B��X��Y��Z�ķ�������Ϊ3�U1�U2 |
C��Z������������Y���������ʵĶ��� |
D����λʱ��������n mol Y��ͬʱ����3n mol X |
��4�����ܱ������ͨ��a mol A��g����b mol B��g����
������ӦA��g��+ B��g��= 2C��g�������ı���������ʱ����ӿ췴Ӧ���ʵ���________������ţ���
�ٽ����¶�
�ڱ���������������䣬���뺤��
�ۼ������
�ܱ���������������䣬����A��g�������ʵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.Mg(OH)2��������Һ�д���ƽ�⣺Mg(OH)2(s)![]() Mg2+(aq)+2OH��(aq)
Mg2+(aq)+2OH��(aq)
B.NaHS��Һ�д��ڵ���ƽ�⣺HS��+H2O![]() H2S+OH-
H2S+OH-
C.NaHCO3��Һ�д���ˮ��ƽ�⣺HCO3��+H2O![]() OH��+H2CO3
OH��+H2CO3
D.��Ӧ��Cr2O72��+ H2O![]() 2CrO42��+2H+����ƽ�ⳣ��K=
2CrO42��+2H+����ƽ�ⳣ��K=
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������ӵ�أ������������¿ɽ���ѭ����ŵ磬ʵ�ֶԴ��ԵĿ�����ء�һ��Ϊ����Fe2O3����һ��Ϊ����﮺�ʯī�ĸ��ϲ��ϣ������ֻ��������ӡ�����ܷ�ӦΪ��Fe2O3+6Li![]() 2Fe+3Li2O�����ڴ˵�أ�����˵������ȷ����
2Fe+3Li2O�����ڴ˵�أ�����˵������ȷ����
A.�ŵ�ʱ���˵����������
B.�ŵ�ʱ��������ӦΪFe2O3+6Li++6e-= 2Fe+3Li2O
C.�ŵ�ʱ������������С��������������
D.���ʱ��������ӦΪLi++e-=Li
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£�CH4��H2O(g)������Ӧ��CH4(g)+H2O(g)![]() CO(g)+3H2(g)������ʼ
CO(g)+3H2(g)������ʼ![]() =Z���ں�ѹ�£�ƽ��ʱ
=Z���ں�ѹ�£�ƽ��ʱ![]() (CH4)�����������Z��T���¶ȣ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
(CH4)�����������Z��T���¶ȣ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
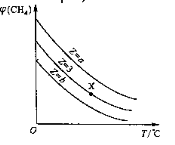
A���÷�Ӧ���ʱ䦤H��0
B��ͼ��Z�Ĵ�СΪb��3��a
C��ͼ��X���Ӧ��ƽ��������![]() =3
=3
D���¶Ȳ���ʱ��ͼ��X���Ӧ��ƽ���ڼ�ѹ��![]() (CH4)��С
(CH4)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)12g̼������ˮ������Ӧ����CO��H2��������131.3KJ�������˷�Ӧ���Ȼ�ѧ����ʽΪ______________________________��
(2)��֪���µ��Ȼ�ѧ��Ӧ����ʽ��
��2CO��g��+O2��g����2CO2��g�� ��H����566kJ/mol
��2H2��g��+O2��g����2H2O��g�� ��H����484kJ/mol
��CH4��g��+2O2��g����CO2��g��+2H2O��g�� ��H����890kJ/mol
��CH4��g��+CO2��g����2CO��g��+2H2��g�� ��H��______��
(3)��֪H2(g)+Br2(l)=2HBr(g) ��H=��102kJ/mol������������������±���
H2(g) | Br2(l) | HBr(g) | |
1mol�����еĻ�ѧ������ʱ��Ҫ���յ�����/kJ | 436 | a | 369 |
�����aΪ_______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com